Human Brain Vascular Pericytes (HBVP)
Cat.No.: CSC-7825W
Species: Human
Source: Brain
Cell Type: Pericyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human Brain Vascular Pericytes (HBVPs) are primarily located along the capillary basement membrane in the central nervous system, closely linked with endothelial cells to form a complex network. Cerebrovascular structures rely on these components to cover the endothelial layer. HBVPs possess smooth muscle-like contractile abilities which help maintain cerebrovascular stability by controlling vascular contraction and dilation and they can be found in tissues with high vascularization such as the placenta. Under certain conditions their pluripotent nature enables them to transform into either neural cells or vascular cells. Neuroscience research utilizes HBVPs to investigate diseases such as Alzheimer's, Parkinson's, and multiple sclerosis while also examining how drugs impact blood-brain barrier stability and vascular operations. The ability of these cells to differentiate between multiple cell types enables them to play crucial roles in neural regeneration and tissue repair processes.
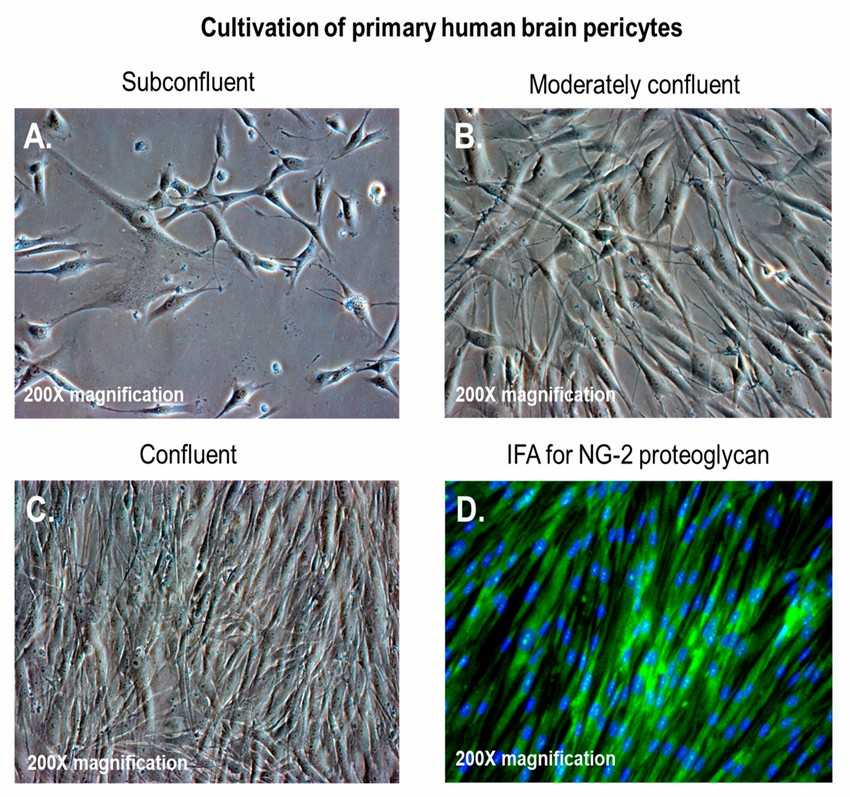 Fig. 1. (A–C) Primary human brain pericytes in culture, from low to high confluency; (D) primary human brain pericytes stained with a monoclonal antibody against neural/glial antigen-2 (NG2) proteoglycan (Alcendor D, 2020).
Fig. 1. (A–C) Primary human brain pericytes in culture, from low to high confluency; (D) primary human brain pericytes stained with a monoclonal antibody against neural/glial antigen-2 (NG2) proteoglycan (Alcendor D, 2020).
PET Micro- and Nanoplastic Particles Affect the Oxygen Consumption Rate (OCR) and Extracellular Acidification (ECAR) of Human Brain Vascular Pericytes
The biological effects of nanoplastics and microplastics create significant health concerns because they spread through environments and food chains before reaching the human body. The ability of these particles to cross the blood-brain barrier leads to oxidative stress and mitochondrial dysfunction. Given that pericytes play a vital role in maintaining the integrity and function of the blood-brain barrier, Gettings et al. evaluated whether polyethylene terephthalate (PET) particles influence these critical brain cells.
Cell counting assay was performed for pericyte exposed to PET for 3, 6 and 10 days. There is a no change of absorbance intensity of pericytes suggesting no change in cell viability following 3 days, 6 days, and 10 days treatment of PET at 50 μg/ml. The Agilent Seahorse XFp Mito Stress Test assessed mitochondrial respiration by measuring OCR (pmol/min) and ECAR (mpH/min). Exposure to PET did not affect ECAR compared to the control regardless of duration, though an interaction was noted at 3 days (Fig. 1A). No significant ECAR changes were observed at 6 days (Fig. 1B) or 10 days (Fig. 1C). However, PET significantly reduced OCR after 3 days of exposure (Fig. 1D), with no significant changes detected at 6 days (Fig. 1E) or 10 days (Fig. 1F).
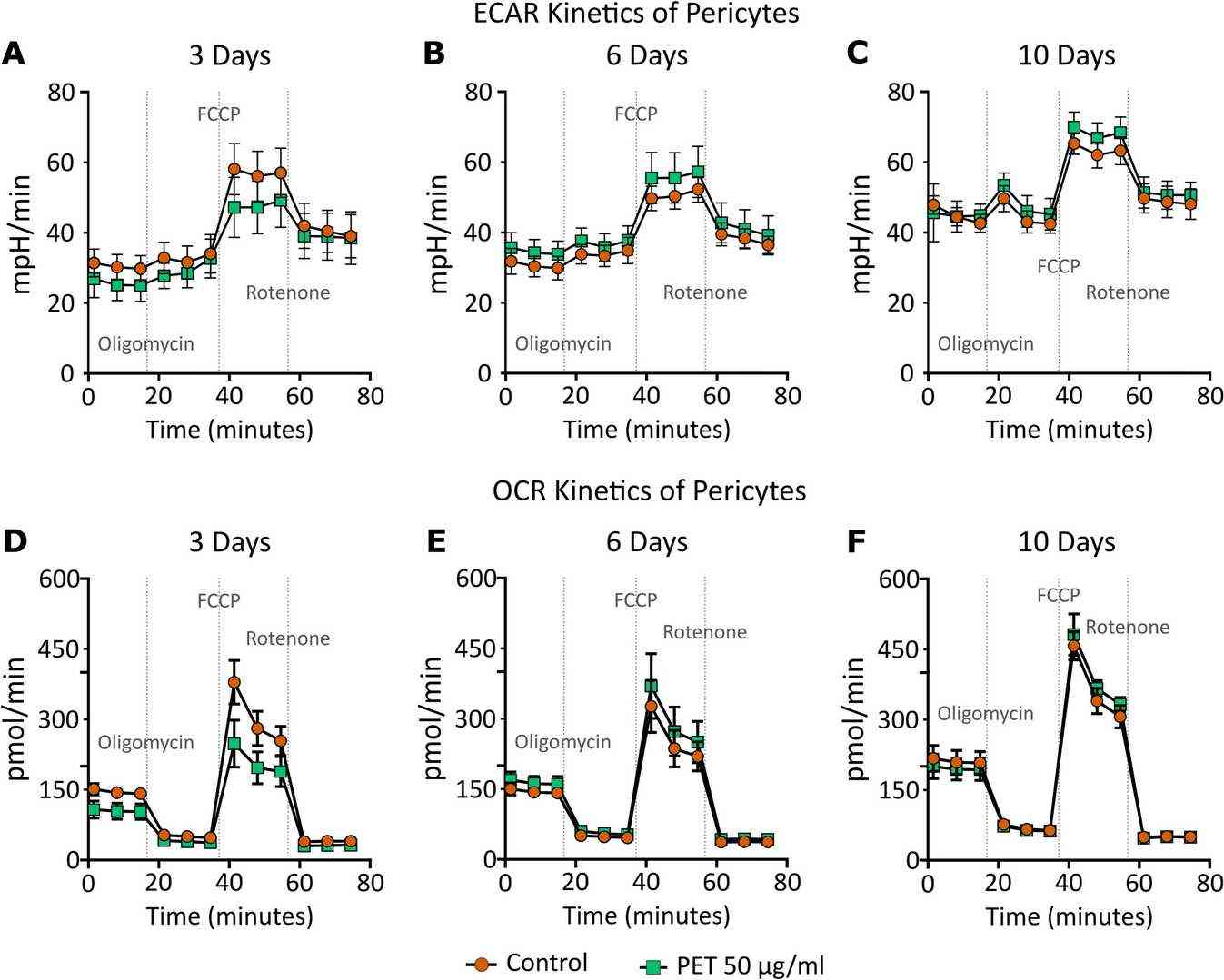 Fig. 1. Extracellular acidification rate (ECAR, A) and oxygen consumption rate (OCR, B) kinetics: ECAR and OCR of pericytes exposed to control (round orange) or 50 μg/ml PET (square green) for 3, 6 and 10 days (Gettings SM, Timburry W, et al., 2024).
Fig. 1. Extracellular acidification rate (ECAR, A) and oxygen consumption rate (OCR, B) kinetics: ECAR and OCR of pericytes exposed to control (round orange) or 50 μg/ml PET (square green) for 3, 6 and 10 days (Gettings SM, Timburry W, et al., 2024).
Effects of oIAPP on HBVP in the Microvascular Model
IAPP aggregates in diabetes and Alzheimer's disease, affecting brain pericytes involved in blood-brain barrier integrity. Despite its association with vascular issues, the impact of IAPP on capillary contraction is less understood. Nu?ez-Diaz et al. uses an in vitro microvasculature model, co-culturing human brain vascular pericytes with endothelial cells, to assess oIAPP's effects on pericyte morphology and contractility.
Co-culturing endothelial cells with HBVP resulted in self-assembled cell clusters connected by branches, forming capillary-like structures (CLS) without a lumen (Fig. 2A, B). These CLS comprised HBVPs closely associated with elongated endothelial cells, with many HBVP located peripherally along the branches (Fig. 2C, D). After 22-hour stimulation with oligomeric IAPP (oIAPP), HBVPs more frequently adopted a round shape (Fig. 2C, D). The proportion of round HBVP significantly increased compared to the vehicle condition, which mostly contained elongated HBVP (Fig. 2C–E). Trypan blue staining showed a low number of dead, round HBVP (<20%), with no significant difference between oIAPP and vehicle treatments (Fig. 2H).
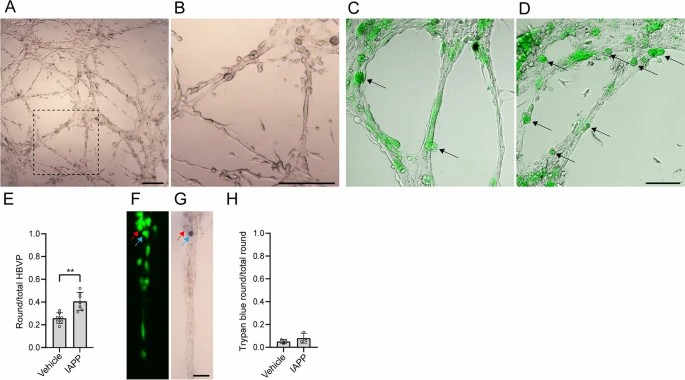 Fig. 2. Effects of oIAPP on HBVP in the microvascular model (Nu?ez-Diaz C, Pocevi?iūt? D, et al., 2023).
Fig. 2. Effects of oIAPP on HBVP in the microvascular model (Nu?ez-Diaz C, Pocevi?iūt? D, et al., 2023).
Ask a Question
Write your own review