Human Bone Marrow-Derived Endothelial Cells
Cat.No.: CSC-C8593W
Species: Human
Source: Bone Marrow
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Bone marrow endothelial progenitor cells (EPCs) serve as the precursors that develop into endothelial cells. As they mature into endothelial cells these cells carry out specific tasks involved in angiogenesis and vascular remodeling. Mesenchymal stem cells situated in the bone marrow maintain their potential to differentiate into endothelial cells. The cells demonstrated significant angiogenic potential in both experimental and living organism studies.
Some studies have also found that endothelial cells originating from bone marrow can mature into vascular endothelial cells while also having the ability to transform into lymphatic endothelial cells (LECs). These cells show angiogenic activity in both inflammatory zones and tumor tissues. Notably, these cells possess not only endothelial functions but also certain immunomodulatory capabilities. For example, in diabetes, hyperglycemia can alter the differentiation direction of these cells, causing them to transform into pro-inflammatory macrophages or dendritic cells. This change in differentiation direction may have significant implications for inflammatory responses and immune regulation.
Plasmodium EVs Deliver RNA Cargo to Human Endothelial Cells
Malaria, caused by Plasmodium falciparum, affects over 400 million people annually, with severe complications like cerebral malaria and severe anemia. Infected red blood cells (iRBCs) release extracellular vesicles (EVs) that facilitate parasite communication and regulate vascular function. These EVs are elevated in severe malaria cases and may serve as biomarkers. However, the exact RNA cargoes in these EVs remain unknown. To explore the RNA content of these EVs, Babatunde et al. isolated EVs from in vitro cultures of P. falciparum-infected red blood cells and performed RNA-Seq.
To understand the role of plasmodial RNAs in cellular communication, they tested their stability and transferability to endothelial cells. Human bone marrow-derived endothelial cells (BMECs) absorbed EVs swiftly as demonstrated in (Fig. 1a). RNA transfer was monitored by qPCR. Endothelial cells demonstrated no plasmodial RNA presence without EVs but showed RNA increase after 12 hours of EV exposure. Endothelial cells can receive plasmodial RNAs via EVs. The transfer process remained unchanged by α-amanitin which blocks RNA Polymerase II and III activity thus proving Plasmodium genes were not actively transcribed in endothelial cells (Fig. 1b). The research team conducted GO analysis to investigate how EV RNAs might function. The research findings indicate that these RNAs take part in RNA binding processes as shown in Figure 1c. The RNAs detected in the EVs show a primary localization pattern in the cytosol and vesicle membranes (Fig. 1c). EV RNAs localize to cytosol or vesicle membranes while they participate in the regulation of translation and gene expression as well as catalytic activity (Fig. 1e). The results indicate that extracellular RNAs could function as regulatory elements in cell communication.
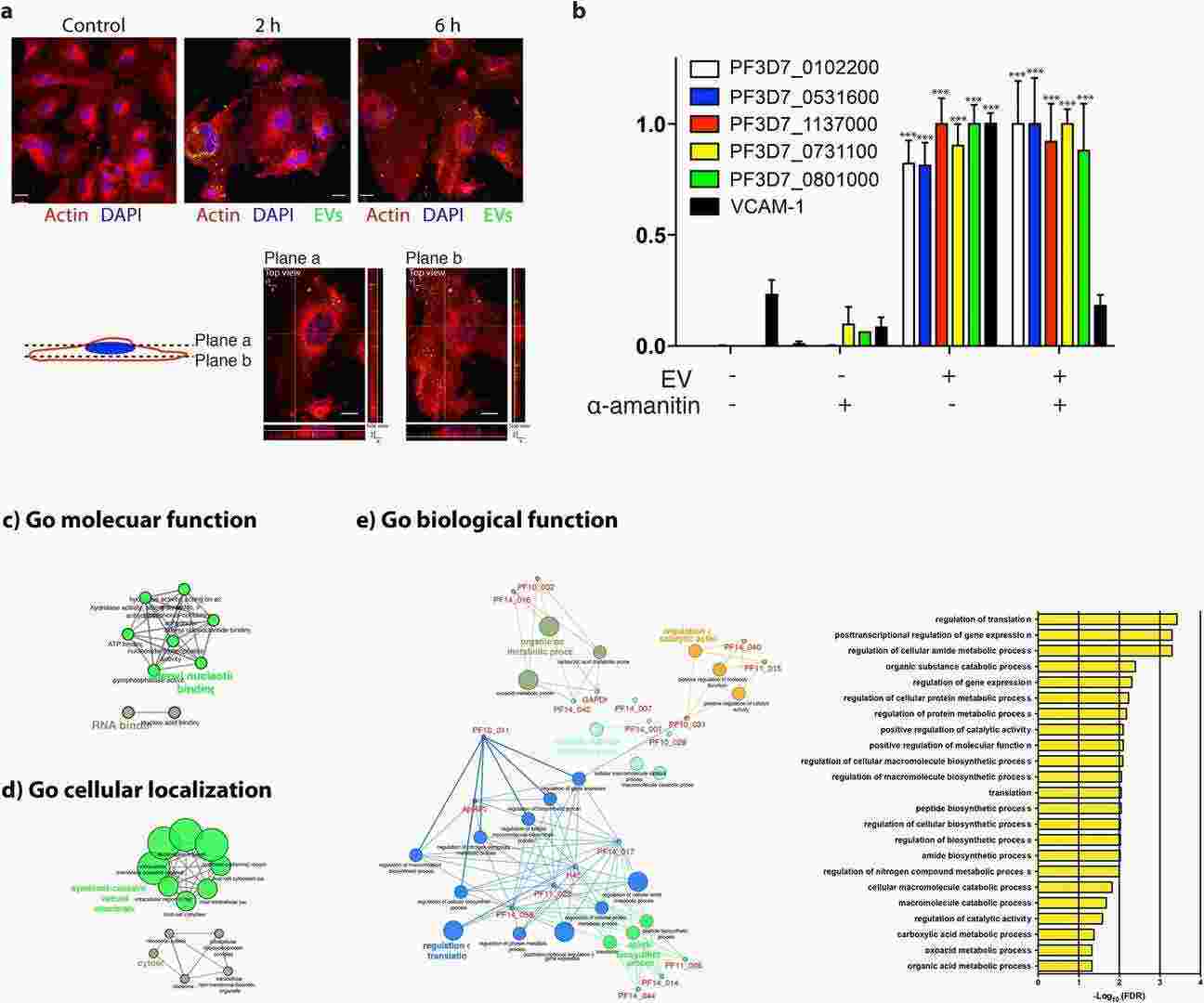 Fig. 1. Transfer of plasmodial RNAs to endothelial cells via EVs (Babatunde KA, Mbagwu S, et al., 2018).
Fig. 1. Transfer of plasmodial RNAs to endothelial cells via EVs (Babatunde KA, Mbagwu S, et al., 2018).
High-throughput Screening of AML-Endothelial Co-Cultures Identifies Anti-Leukemic Compounds
Acute myeloid leukemia (AML) is characterized by refractory disease, often originating from vascular-associated AML cells. Vijay et al. aim to identify novel therapeutic targets for AML. To identify vulnerabilities in vascular-associated AML cells, they screened a synthetic chemical library (SCL) of over 30 million compounds in a co-culture assay using human AML cells (KG1) on a layer of bone marrow-derived endothelial cells (BMECs). The same libraries were counter-screened with CD4+ T lymphocytes. SCLs highly toxic to BMECs or CD4+ T lymphocytes were discarded to avoid vasculotoxic or immunosuppressive agents (Fig. 2A). Eight SCLs selectively killed AML cells (>50% toxicity) while sparing BMECs and causing minimal toxicity to CD4+ T lymphocytes (<30% cell death) (Fig. 2B). These SCLs were further analyzed by deconvolution and positional scanning to define their structure-activity relationship (SAR) for AML cell death and BMEC disruption. SCLs 1953 and 2210 had the highest number of AML-toxic compounds while sparing BMECs, guiding the synthesis of subsequent SCLs 2454, 2470, and 2476.
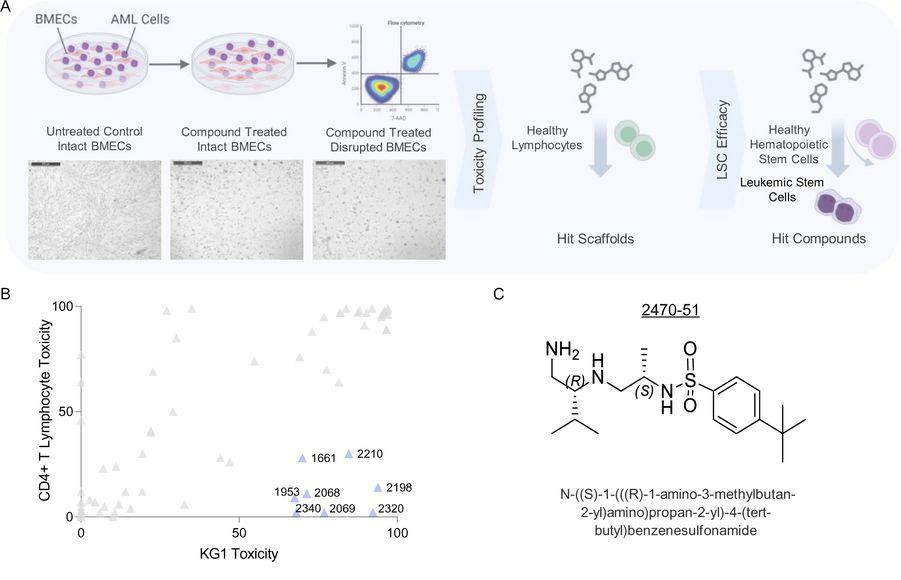 Fig. 2. High-throughput chemical-phenotypic screening using AML-endothelial cell co-culture assay identifies anti-leukemic compounds selectively toxic to AML cells in the vascular niche (Vijay V, Meacham A, et al., 2023).
Fig. 2. High-throughput chemical-phenotypic screening using AML-endothelial cell co-culture assay identifies anti-leukemic compounds selectively toxic to AML cells in the vascular niche (Vijay V, Meacham A, et al., 2023).
The method we use to isolate primary endothelial cells was developed based on a combination of established and our proprietary methods. These cells are isolated with anti-PECAM-1 antibody-conjugated magnetic beads.
Primary endothelial cells produced from Creative Bioarray display typical spindle morphology/or cobblestone morphology under light microscopy and show VE-cadherin (CD144) or CD31 staining at cell-cell junction as demonstrated by fluorescence staining.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Good growth
Cells grow well in the medium recommended by the product.
09 Jan 2021
Ease of use
After sales services
Value for money
Write your own review