Rabbit Corneal Epithelial Cells
Cat.No.: CSC-C9263J
Species: Rabbit
Source: Cornea; Eye
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rabbit corneal epithelial cells (RCECs) are obtained by extracting them from rabbit corneal epithelium. The cornea serves as a transparent protective tissue located at the eye's front and consists of five layers where the outermost one is called corneal epithelium. The outermost corneal epithelium layer contains tightly packed stratified squamous cells. Corneal epithelial cells are highly specialized and play a crucial role in maintaining corneal health through their unique structural and functional attributes. RCECs are vital in preserving the cornea's protective barrier and transparency. By secreting mucins and other protective substances, these cells keep the cornea moist and shield it from microbial invasions and harmful substances. RCECs also function as essential repair cells in the cornea by quickly multiplying to mend damaged regions after sustaining an injury.
RCECs represent essential components for studies in eye research as well as regenerative medical applications. Researchers use them extensively to investigate how the cornea heals after damage occurs. For instance, the effectiveness of various treatment strategies can be tested through the transplantation of cultured RCECs into rabbit eye models. Additionally, RCECs serve as building blocks for creating artificial corneal tissues. Cultured RCECs develop into corneal epithelial layers which allow them to serve as transplant material or as a repair substance for injured corneas.
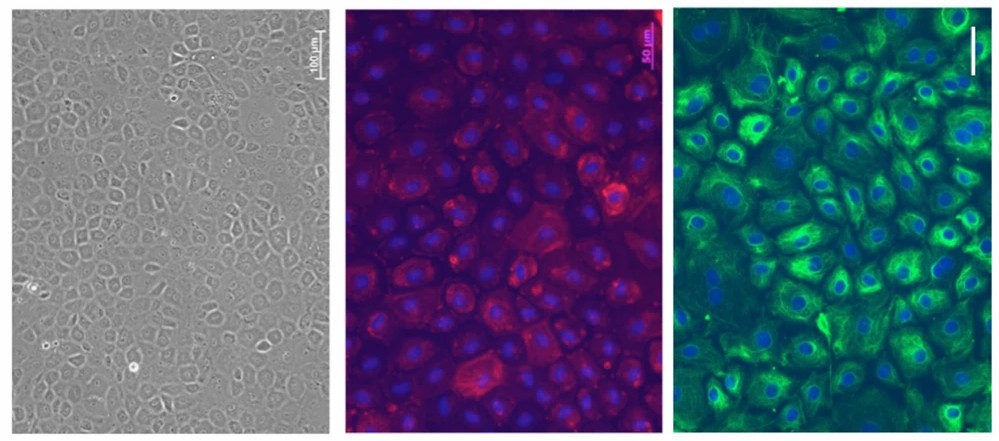 Fig. 1. Morphology of Fresh RCECs (left). Immunohistochemistry of the fibroblastic markers α-SMA (middle) and vimentin (right) (Yamashita K, Hatou S, et al., 2018).
Fig. 1. Morphology of Fresh RCECs (left). Immunohistochemistry of the fibroblastic markers α-SMA (middle) and vimentin (right) (Yamashita K, Hatou S, et al., 2018).
Corneal Fibroblasts and Corneal Epithelial Cells Coordinate in Epithelial BM Assembly
The corneal epithelial basement membrane supports ocular function by providing structural integrity and guiding repair processes through interactions with fibroblasts and myofibroblasts after injury. The cellular interactions in this context prevent excessive fibrosis and preserve corneal transparency which both play a crucial role in sustaining vision. Shiju et al. identified which cell types contribute to the assembly of the corneal epithelial BM during wound healing, focusing on the coordination between epithelial cells and corneal fibroblasts.
They employed a 3D corneal organotypic model and a rabbit photorefractive keratectomy (PRK) model to study the BM assembly. The 3D corneal organotypic model was established by culturing the rabbit corneal epithelial cells with either corneal fibroblasts or myofibroblasts embedded in collagen type I for 18 days. Figure 1A shows a comparison of 3D cultures among corneal fibroblasts with epithelial cells (CFib + Epi), corneal myofibroblasts with epithelial cells (CMyo + Epi), and epithelial cells alone (Epi only controls) for BM markers: laminin alpha-5, perlecan, and nidogen-1. Figure 1B represents the percentage assembly of these BM components. CFib + Epi cultures formed a complete epithelial BM with all three markers, showing about 90% coverage at the epithelial-stromal interface. Specifically, layers of laminin alpha-5, perlecan, and nidogen-1 were 93%, 96%, and 92% complete, respectively. In contrast, CMyo + Epi cultures failed to assemble a proper epithelial BM, forming only 5% laminin alpha-5 and 2% nidogen-1, despite 20% perlecan presence due to its expression by myofibroblasts. Epithelial cells alone formed 69% laminin alpha-5, 49% perlecan, and no nidogen-1.
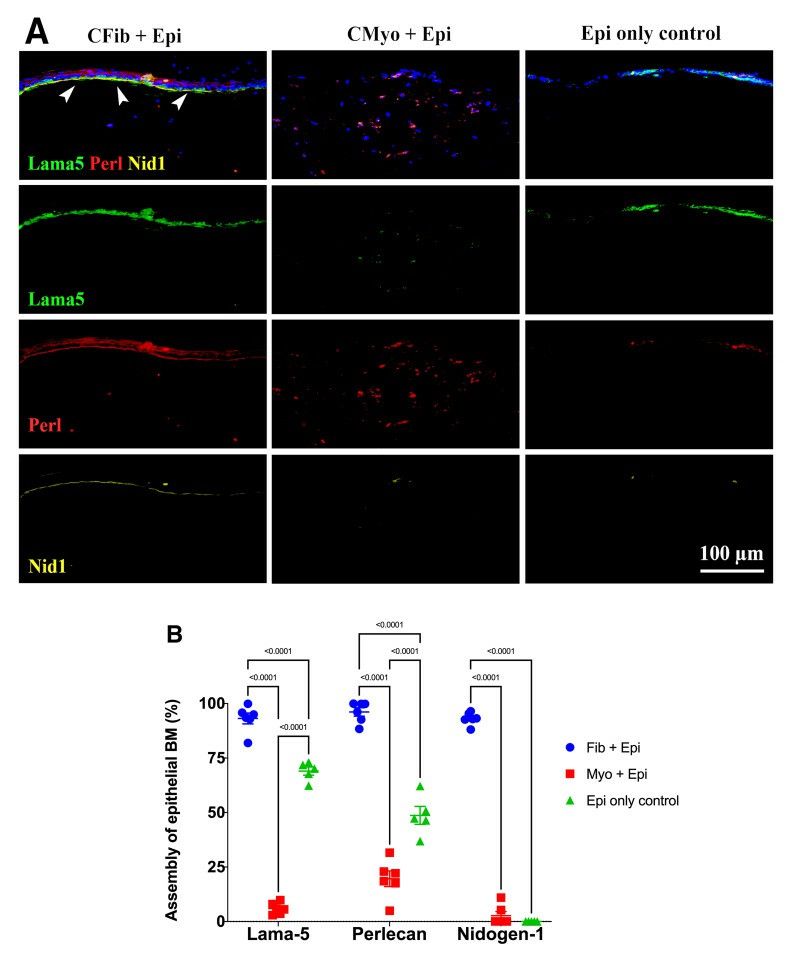 Fig. 1. Comparison of laminin alpha 5, perlecan and nidogen-1 expression in different 3D cultures (Shiju T M, Sampaio L P, et al., 2023).
Fig. 1. Comparison of laminin alpha 5, perlecan and nidogen-1 expression in different 3D cultures (Shiju T M, Sampaio L P, et al., 2023).
RCECs Cultivated on OEM Retain Better Cell Morphology and Function than those Cultured on PETM
Maintaining corneal epithelial layers is vital, relying on LSCs for regeneration. Deficiency in these cells can impair vision. Traditional scaffolds like amniotic membranes support epithelial growth but have limitations. Plant-based scaffolds, like onion epithelial membranes, offer a transparent, accessible alternative.
Wang's team explored the use of onion epithelial membranes (OEM) as a scaffold for cultivating corneal epithelial cells. It aims to evaluate if OEM can effectively support the proliferation and restoration of corneal epithelium in limbal stem cell-deficient models as a cost-effective and accessible alternative. Corneal epithelial cell lines, notably human corneal epithelial cells and mouse corneal epithelial stem/progenitor cell line (TKE2), successfully adhered to and thrived on the OEM surface. To evaluate OEM's effectiveness as a tissue-engineering scaffold, rabbit corneal epithelial cells (RCECs) were cultured on: 1) OEM inserts; 2) Collagen-coated OEM inserts; 3) PETM inserts. After 3 days, collagen coating did not enhance cell adherence on OEM (Fig. 2A). By day 5, all groups reached nearly 100% confluence. The OEM and collagen-coated OEM groups displayed polygonal cell shapes typical of in vivo corneal epithelium, whereas PETM group cells became fusiform or round. By day 7, OEM groups maintained their morphology, while PETM group lost typical features. SEM images showed OEM groups had more microvilli than PETM, indicating better tear adhesion potential (Fig. 2B). The EdU assay results indicated no significant difference in proliferation rates among the three cultured groups up to day five (Fig. 3A and B). However, differences appeared in cloned cultures later. The OEM group had larger clone volumes (Fig. 3C) and significantly higher colony forming efficiency (CFE) compared to the other groups (Fig. 3D). RCECs on OEM demonstrated superior cell morphology and function than those on PETM, suggesting OEM is a better scaffold for transferring corneal epithelial cultures for reconstruction surgery.
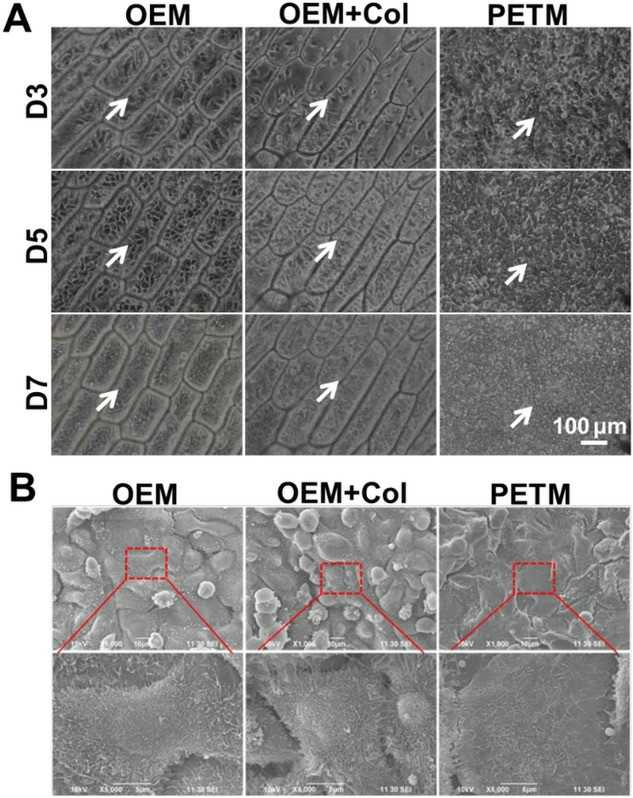 Fig. 2. RCECs cultivated on OEM retain better cell morphology and function than those cultured on PETM (Wang G, Chen P, et al., 2020).
Fig. 2. RCECs cultivated on OEM retain better cell morphology and function than those cultured on PETM (Wang G, Chen P, et al., 2020).
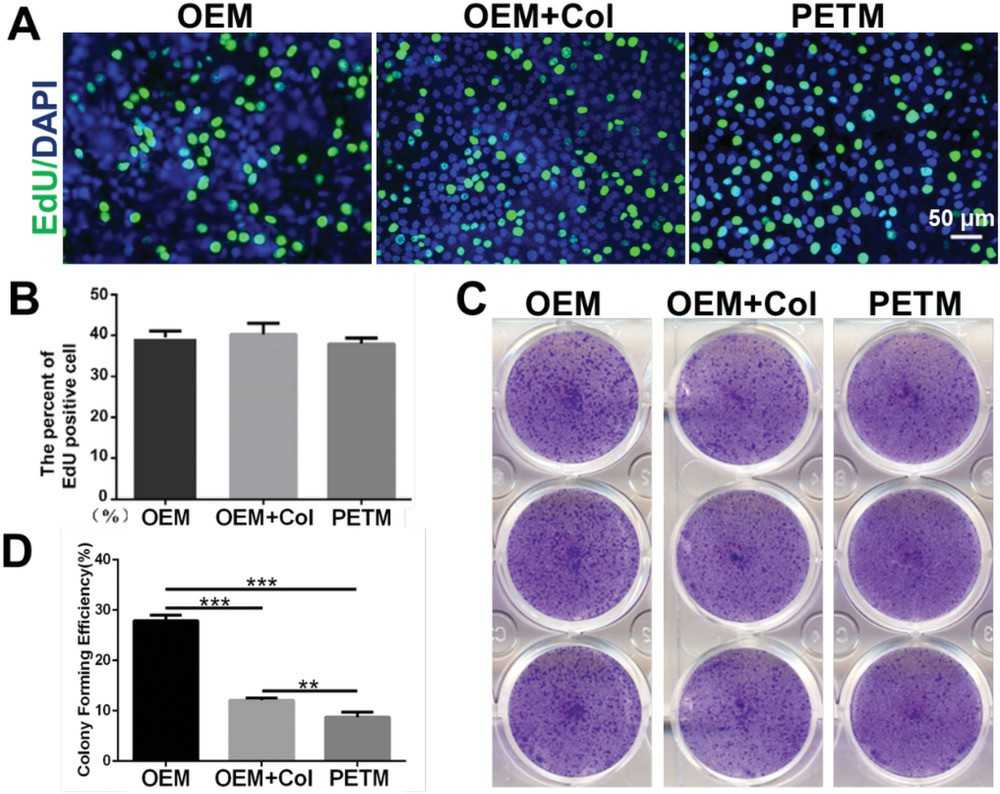 Fig. 3. RCECs cultivated on OEM were superior in cell proliferative capacity than those cultured on PETM (Wang G, Chen P, et al., 2020).
Fig. 3. RCECs cultivated on OEM were superior in cell proliferative capacity than those cultured on PETM (Wang G, Chen P, et al., 2020).
Ask a Question
Write your own review

