Human Normal Peripheral Blood Mononuclear Cells (PBMCs)
Cat.No.: CSC-7652W
Species: Human
Source: Peripheral Blood; Blood
Cell Type: Mononuclear Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Samples from each donor are tested via PCR to confirm non-reactivity.
Human peripheral blood mononuclear cells (PBMCs) are an important group of immune cells in the blood. Lymphocytes make up 70-90% of PBMCs which includes T cells at 70-85%, B cells at 5-10%, and NK cells at 5-20%; monocytes account for 10-20%; and dendritic cells represent only 1-2%. Each of these cells performs specialized roles in maintaining immune system functions. T cells function in cellular immunity and B cells generate antibodies while natural killer (NK) cells destroy infected cells directly and monocytes together with dendritic cells perform antigen presentation and initiate inflammatory responses.
Recent studies have shown that PBMCs not only possess immune functions but also have the potential for multi-directional differentiation, enabling them to transform into various cell types including smooth muscle cells (SMCs), cardiomyocytes, osteoblasts and osteoclasts, nerve cells and induced pluripotent stem cells. Therefore, they play an important role in the fields of cell therapy and personalized medicine. PBMCs also serve as the primary sources for CAR-T cell therapy which has shown effectiveness in treating hematological malignancies like B-cell lymphoma and leukemia as well as solid tumors including lung cancer and ovarian cancer.
Photodynamic Effects with 5-Aminolevulinic Acid on Cytokines and Exosomes in Human Peripheral Blood Mononuclear Cells
Photodynamic therapy (PDT) using 5-aminolevulinic acid (ALA) is a well-established treatment for various malignant and premalignant diseases. It works by generating reactive oxygen species (ROS) through light-activated protoporphyrin IX (PpIX), causing apoptosis and necrosis in targeted lesions. However, the effects of ALA-PDT on exosome levels and cytokines in peripheral blood mononuclear cells (PBMCs) are poorly understood.
Espeland et al. investigated the impact of ALA-PDT on cytokines and exosomes in healthy human PBMCs using electron microscopy and flow cytometry. Figure 1 shows PpIX production in PBMC subpopulations from four donors after 4 h of 3 mM ALA incubation in the dark. Most PBMC subsets produced PpIX at 200%–300% of control levels without ALA. However, CD8+ T cells produced little PpIX, while CD3−CD19+ B cells showed higher PpIX levels with significant variation among donors. No obvious dark toxicity was observed in various PBMC subpopulations (Fig. 2), although some variation existed. Notably, CD3−CD56+ NK cells exhibited cytotoxicity with ALA alone (Fig. 2). Generally, 3 mM ALA for 4 h does not cause cytotoxicity. However, the extended 4-h ALA incubation followed by 48 h for cytokine and exosome preservation might induce toxicity in certain PBMC subsets.
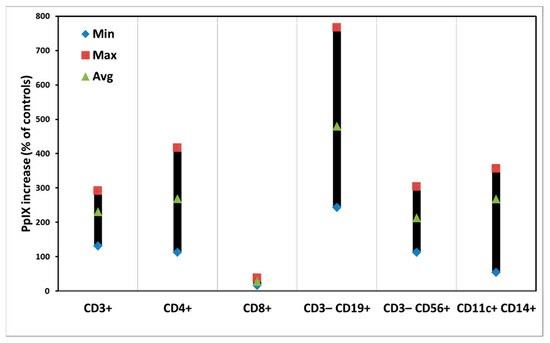 Fig. 1. Intracellular amounts of PpIX in individual subsets of PBMCs after ALA incubation for 4 h (Espeland K, Kleinauskas A, et al., 2022).
Fig. 1. Intracellular amounts of PpIX in individual subsets of PBMCs after ALA incubation for 4 h (Espeland K, Kleinauskas A, et al., 2022).
 Fig. 2. Dark toxicity of cells in individual subsets of PBMCs after ALA incubation for 52 h. The data are presented as minimal, maximal, and average values (Espeland K, Kleinauskas A, et al., 2022).
Fig. 2. Dark toxicity of cells in individual subsets of PBMCs after ALA incubation for 52 h. The data are presented as minimal, maximal, and average values (Espeland K, Kleinauskas A, et al., 2022).
Cytotoxicity and Compatibility Investigations of In₂O₃ nanoparticles
Nanocomposites combining metal oxides with graphene have shown promise in various applications. Alaizeri et al. investigated the effects of RGO doping on the photocatalytic and anticancer properties of In₂O₃ nanoparticles. The pure and doped In₂O₃ nanoparticles were synthesized using a microwave-assisted hydrothermal process. Characterization was performed using XRD, TEM, SEM, EDX, XPS, Raman, UV–Vis, and PL spectroscopy.
Then, they tested the cytotoxicity and compatibility using HC116, HepG2 and normal peripheral blood mononuclear cells (PBMCs). Figure 3A indicates that cell viability of HC116 cells decreased with increasing sample concentrations. Similarly, cytotoxicity against HepG2 cells increased with higher concentrations. These results demonstrate that In₂O₃/RGO NCs have greater cytotoxicity towards HC116 and HepG2 cancer cells than pure In₂O₃ NPs. Importantly, Figure 3C illustrates that the viability of normal PBMCs remained stable across all concentrations, indicating good cytocompatibility. The findings suggest that RGO enhances the anticancer efficacy of In₂O₃ NPs.

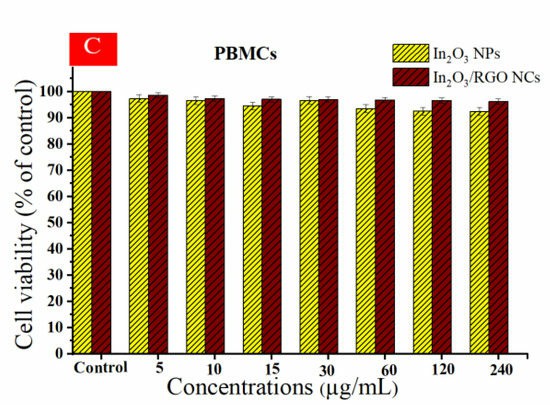 Fig. 3. Cell viability of HC116 cell line (A), HepG2 cells (B), and the cytocompatibility of prepared samples in human peripheral blood mononuclear cells (PBMCs) (C) (Alaizeri ZM, Alhadlaq HA, et al., 2023).
Fig. 3. Cell viability of HC116 cell line (A), HepG2 cells (B), and the cytocompatibility of prepared samples in human peripheral blood mononuclear cells (PBMCs) (C) (Alaizeri ZM, Alhadlaq HA, et al., 2023).
Ask a Question
Write your own review