- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Tumorigenecity: grow in BALB/c mouse
Histopathology: breast cancer
Note: MTV-L/CL2 (KCLB 60056) mouse mammary tumor cells fused with thymocyte and macrophages, it metastasizes to the lung massively and causes cachexia, well differentiated.
Shipping Condition: Room Temperature
MTV/TM-011 was isolated from the mammary gland tissue of BALB/c mice, and its original tumor type was breast cancer lesion. In the in vitro culture system, this cell line generally presents with the adherent epithelial-like cell morphology (irregular cell shape, tight intercellular junction and monolayer sheet-like growth). Microenvironmental signals from 3T3-L1 adipocytes enable MTV/TM-011 cells to separate from their two-dimensional adherence and develop three-dimensional spheroid structures. As this morphological change well recapitulates the 3D growth pattern of solid tumor in vivo and provides a more convenient visual representation, the model is often used for studying their invasion and metastasis.
The cell line shows high rates of pulmonary metastasis and cachexia induction, making it widely used in breast cancer research studies. On one hand, it was extensively used to study the role of some key molecules (including GREM1, STAT3, MMP13, etc.) in the process of invasion-metastasis cascade. On the other hand, based on the adipocyte co-culture system, this model was also used to reveal the regulation of adipocyte-secreted factors (such as IL-6) on breast cancer cell proliferation and migration. Moreover, this model was heavily used for screening of anticancer drugs and testing of therapeutic strategies, in which candidate compound or combination therapies could be verified to inhibit breast cancer cell proliferation, block invasion, and prevent distant metastasis.
Grem2-overexpressing Adipocytes Inhibit Proliferation and Invasion of Breast Cancer Cells
Gremlin-2 (GREM2), a bone morphogenetic protein antagonist, is known to inhibit adipogenesis but its role in cancer remains unclear. Unlike Gremlin-1, which promotes cancer, GREM2's function in tumor progression is not well understood. Jung et al. investigated whether GREM2's suppression of adipogenesis affects breast cancer cell growth and metastasis.
They used a 3D co-culture system to assess how GREM2-suppressed adipogenesis affects breast cancer cells. 3T3-L1-mock and 3T3-L1-Grem2 cells were differentiated into adipocytes for 9 days, then mixed with MTV/TM-011 breast cancer cells to form 3D spheroids (Fig. 1a). Fluorescent staining (red: cancer cells, green: adipocytes) confirmed even cell distribution. Spheroids with adipocytes-Grem2 were smaller (Fig. 1b) and showed reduced proliferation (Fig. 1c) compared to those with adipocytes-mock. Invasion assays (Fig. 1d) revealed that spheroids containing adipocytes-Grem2 had significantly smaller invasion areas on days 3, 7, and 9 (Fig. 1e, f). These findings indicate that Grem2-overexpressing adipocytes inhibit breast cancer cell proliferation and invasion.
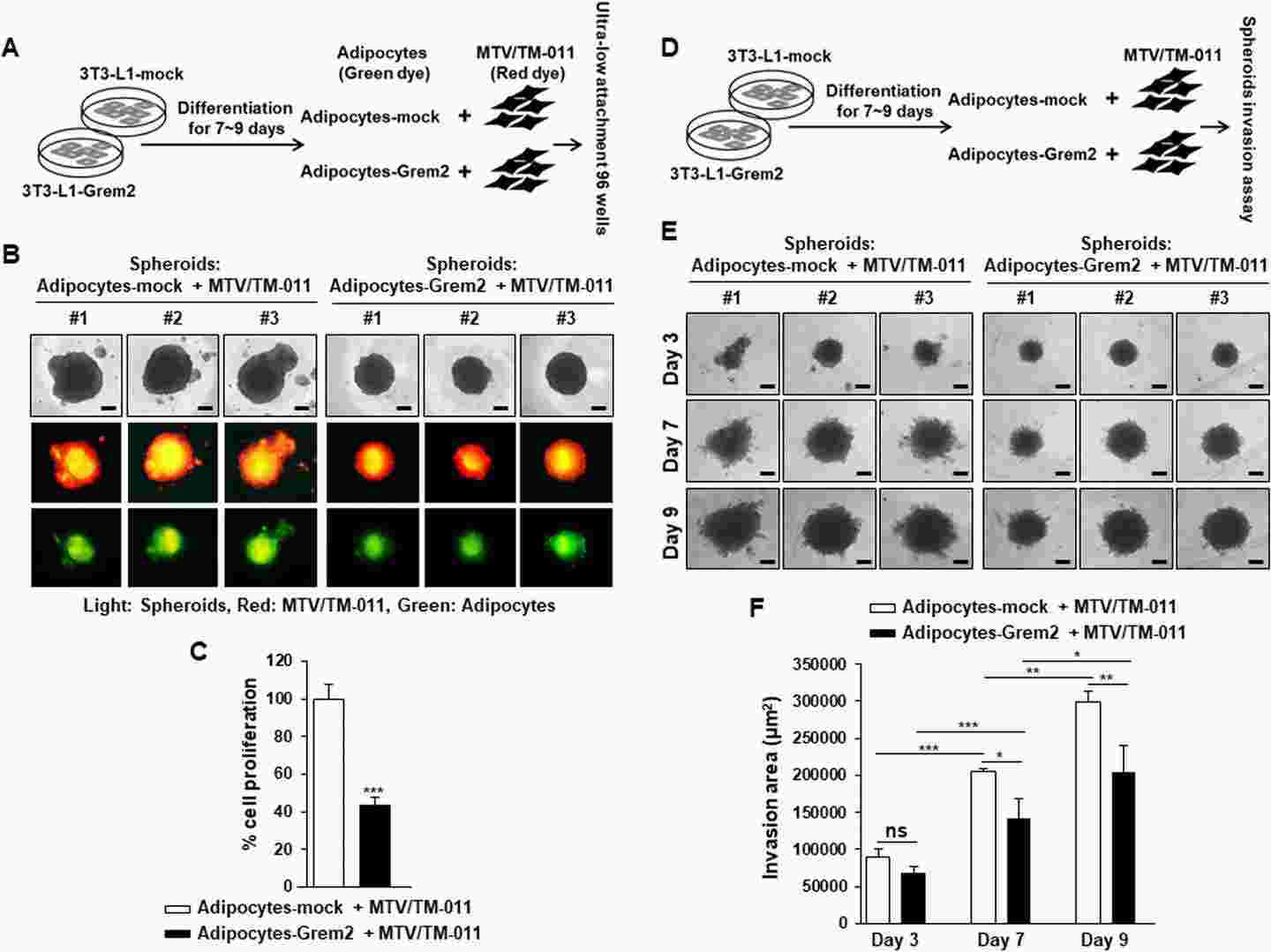 Fig. 1. Adipocytes overexpressing Grem2 inhibit the proliferation and invasion of breast cancer cells (Jung J, Kim N H, et al., 2023).
Fig. 1. Adipocytes overexpressing Grem2 inhibit the proliferation and invasion of breast cancer cells (Jung J, Kim N H, et al., 2023).
GREM1 Contributes to the Proliferation, Migration, and Invasion of Breast Cancer Cells
The BMP antagonist Gremlin-1 (GREM1) is overexpressed in breast cancer and associated with metastasis, however the mechanism is not understood. Using in vitro assays and an orthotopic breast cancer mouse model Sung et al. examined the effect of knockdown and overexpression of GREM1 on breast cancer cell proliferation, migration and invasion. In order to validate the requirement for GREM1 in breast cancer cell growth and migration, they silenced GREM1 in MDA-MB-231 and MTV/TM-011 cells via lentiviral transduction (Fig. 2A). They found that knockdown of GREM1 significantly decreased cell proliferation (Fig. 2B) and migration as determined by wound healing assay (Fig. 2C–F). In contrast, transient overexpression of GREM1 (Fig. 3A) resulted in significantly increased proliferation (Fig. 3B), migration (Fig. 3C–F) and invasion (Fig. 3G and H) as compared to control. Taken together, their data indicates a role for GREM1 in breast cancer cell growth, migration and invasion.
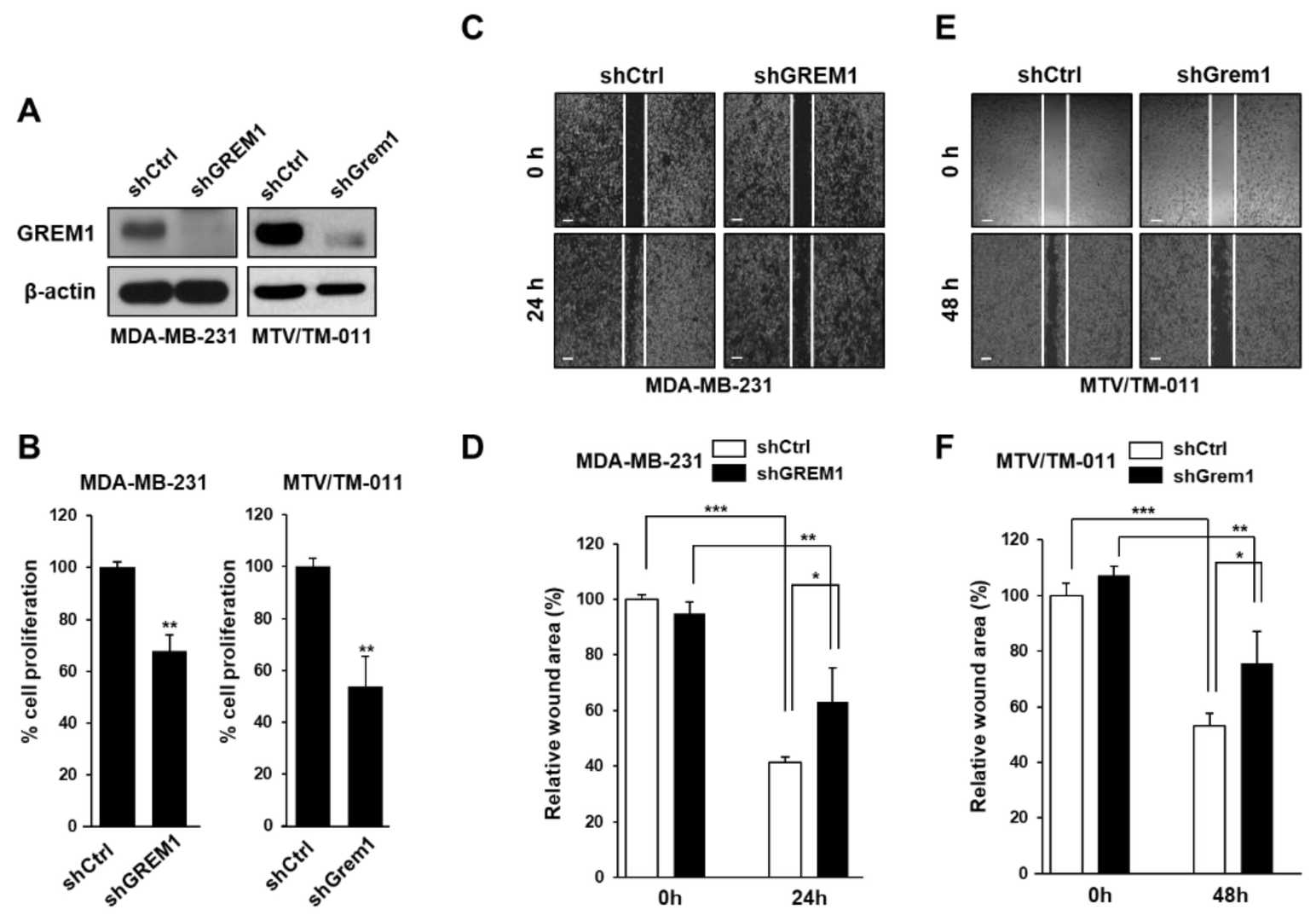 Fig. 2. GREM1 knockdown inhibits the growth and mobility of breast cancer cells (Sung NJ, Kim NH, et al., 2020).
Fig. 2. GREM1 knockdown inhibits the growth and mobility of breast cancer cells (Sung NJ, Kim NH, et al., 2020).
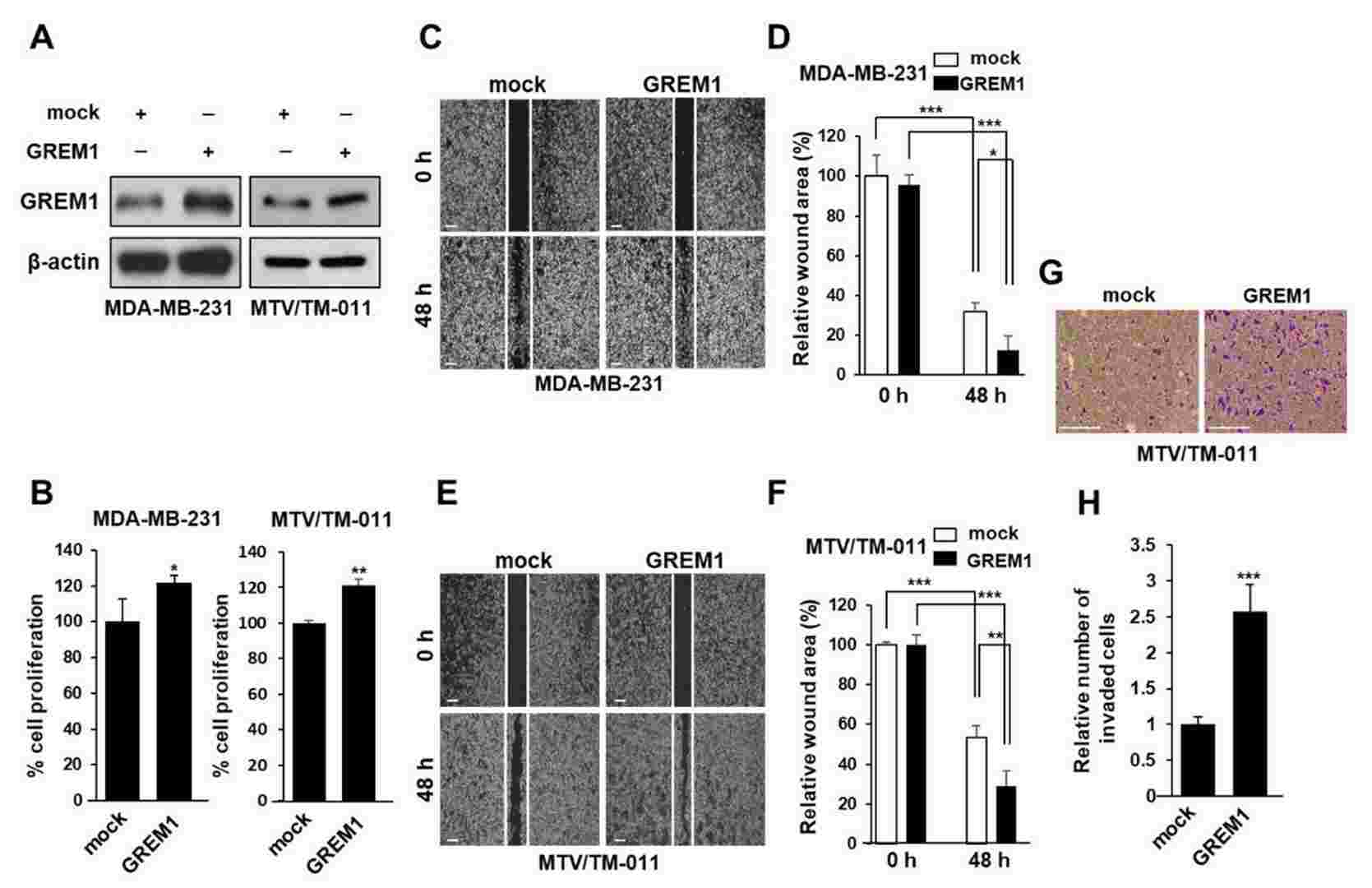 Fig. 3. GREM1 overexpression enhances the growth and mobility of breast cancer cells (Sung NJ, Kim NH, et al., 2020).
Fig. 3. GREM1 overexpression enhances the growth and mobility of breast cancer cells (Sung NJ, Kim NH, et al., 2020).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells