Human Lymphatic Endothelial Cells
Cat.No.: CSC-C1497
Species: Human
Source: Lymph Node
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
HLEC are isolated from human lymph nodes. HLEC are cryopreserved on passage one and delivered frozen. Each vial contains >5 x 10 ^5 cells in 1 ml volume. HLEC are characterized by immunofluorescent method with antibodies to vWF/Factor VIII and CD31 (P-CAM) and by uptake of DiI-Ac-LDL. HLEC are negative for HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi. HLEC are guaranteed to further expand for 10 population doublings at the conditions provided by Creative Bioarray.
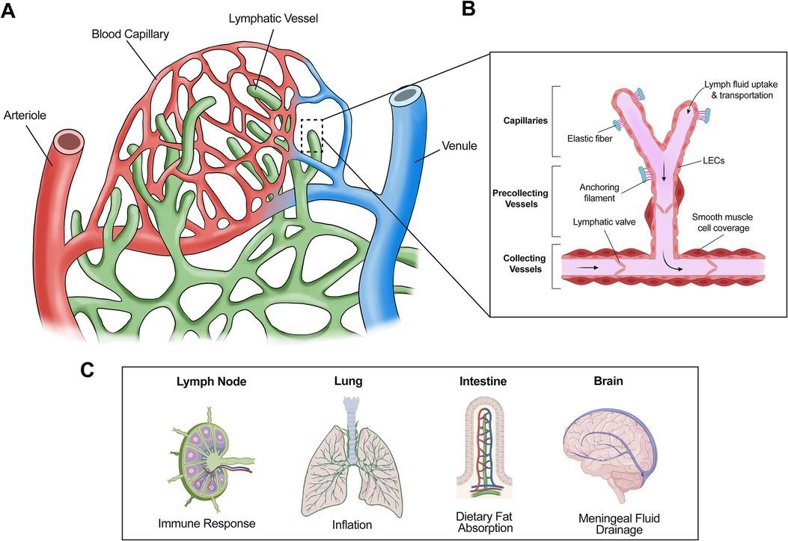
Human Lymphatic Endothelial Cells (HLEC) are a type of endothelial cell that line the interior surface of lymphatic vessels. They can be obtained through biopsies of human lymph nodes and skin dermis, as they are a crucial component of the lymphatic system, which is responsible for the transport of lymph fluid, immune cell trafficking, and immune response. HLEC have distinct morphological characteristics, including thin-walled vessels with numerous valves and fenestrations, and play a role in facilitating lymph flow and immune surveillance. In culture, HLEC require specific growth conditions, including fibronectin-coated surfaces and specialized media to maintain viability. Human Lymphatic Endothelial Cells (HLEC) are a type of endothelial cell that line the interior surface of lymphatic vessels.
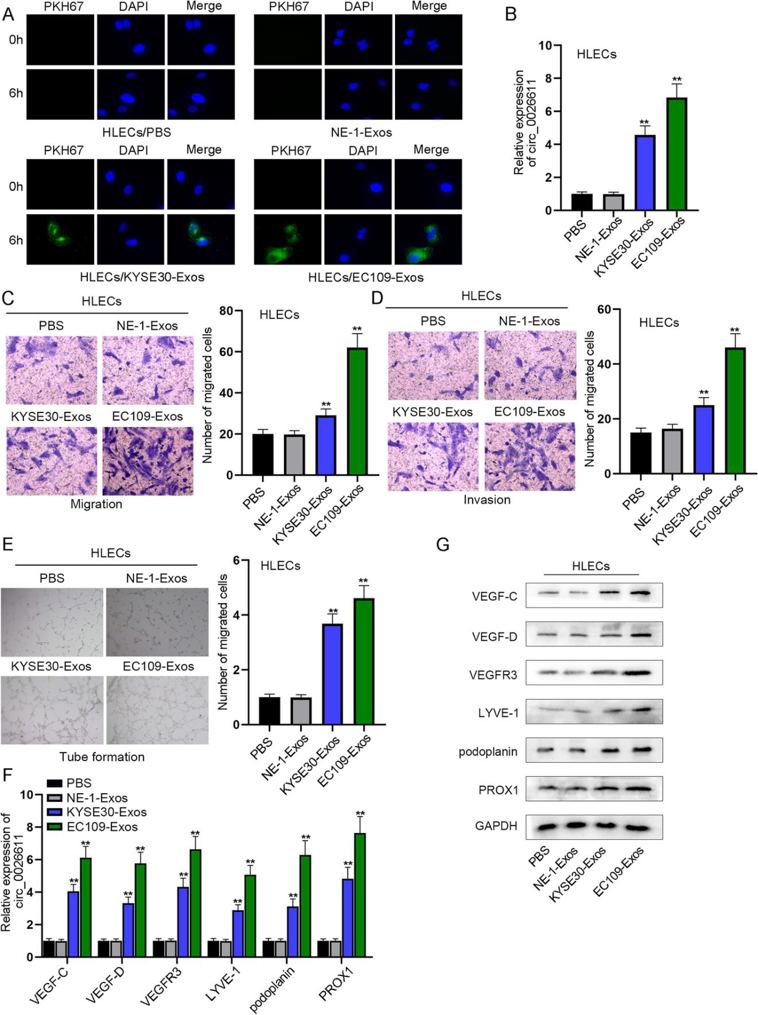
ESCC Cell-Derived Exosomes Promoted the Migration, Invasion, and Lymphangiogenesis of HLECs
Esophageal squamous carcinoma (ESCC) is a common malignancy with late-stage diagnosis and frequent recurrence. Lymph node metastasis (LNM) is a typical sign of ESCC treatment failure. Lymphangiogenesis is associated with LNM in ESCC, but the underlying mechanisms remain unclear. In this study, Yao’s team analyzed the expression of circ_0026611 in ESCC cells and exosomes by RT-qPCR, and explored the effect and mechanism of circ_0026611 on lymphangiogenesis.
Exosome labeling and tracking assay showed that ESCC exosomes could be absorbed by human lymphatic endothelial cells (HLECs). After 6 h of culture, PKH67-labeled exosomes from ESCC cells were internalized by HLECs (Fig. 1A). The expression of circ_0026611 in HLECs was significantly upregulated after absorbing exosomes from KYSE30. In addition, after HLECs absorbed exosomes from EC109, circ_0026611 was more significantly upregulated (Fig. 1B). Migration and invasion of HLECs after KYSE30-Exos treatment were significantly enhanced, while the effects were more significant after EC109-Exos treatment (Fig. 1C, D). Tube formation assay showed that KYSE30-Exos treatment promoted the tube formation ability of HLECs, and EC109-Exos had a stronger effect (Fig. 1E). The expression of lymphangiogenesis-related markers (VEGF-C, VEGF-D, VEGFR3, LYVE-1, podoplanin, PROX1) was significantly upregulated in HLECs after KYSE30-Exos treatment, while the effects were more significant after EC109-Exos treatment (Fig. 1F, G). Therefore, exosomes secreted by ESCC cells promote lymphangiogenesis.
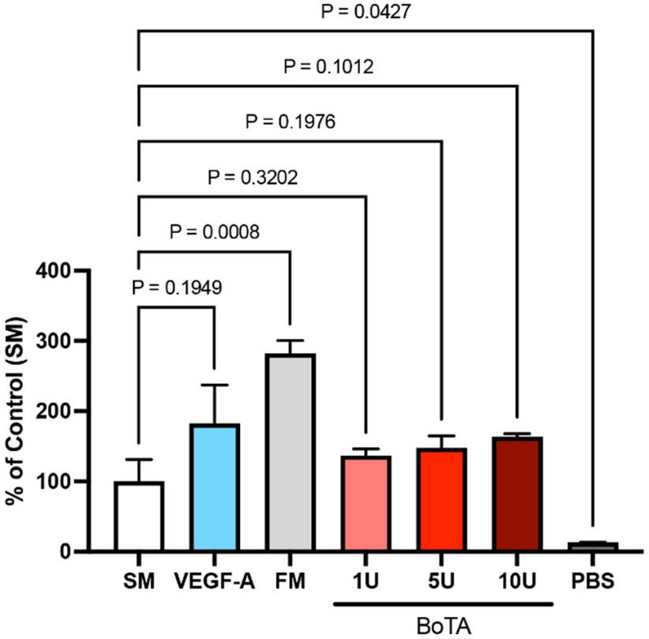
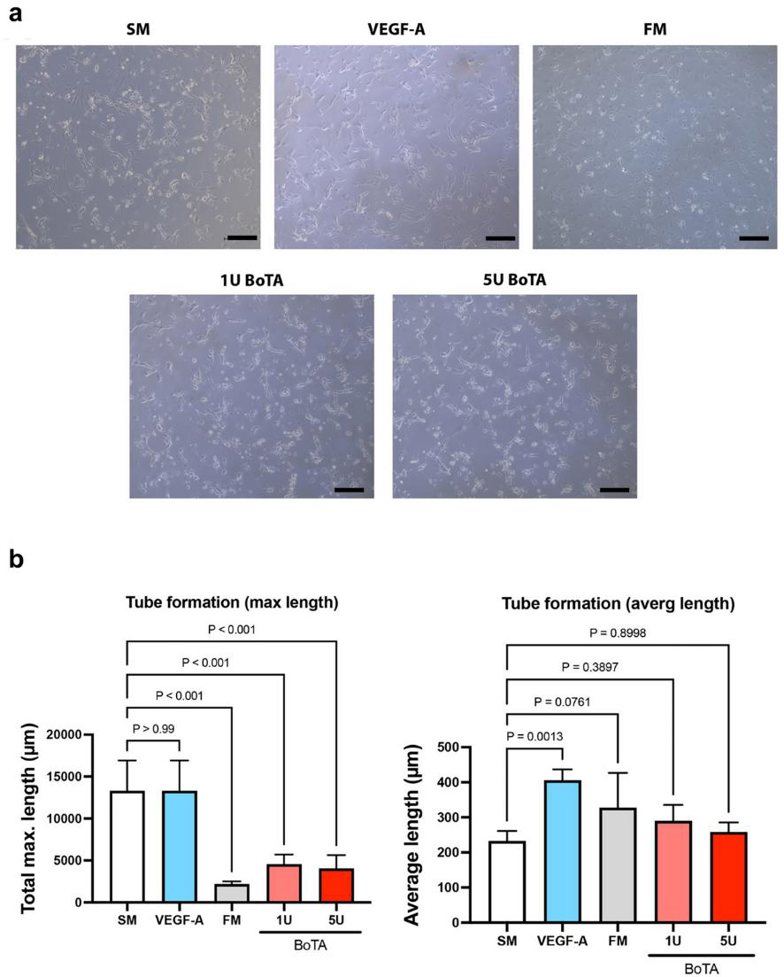
BoTA Influences Lymphangiogenesis by Significantly Decreasing the Maximum Tube Length of Cultured LECs
Botulinum toxin A (BoTA) is a neurotoxin with a wide range of medical applications, primarily affecting muscles. Its effects on other systems, including blood vessels, have been studied, but its impact on the lymphatic vascular system remains unknown. Vasella et al. evaluated the effects of BoTA on human lymphatic endothelial cells (LECs) using in vitro culture systems and various analytical techniques.
To determine the direct influence of BoTA on LECs, they compared LEC proliferation after applying different BoTA dosages, and different controls (starvation medium, full medium, VEGF-A medium in starvation medium, and PBS). LEC proliferation was significantly higher in full medium than in any other medium, while PBS was the worst. However, BoTA dosages, independent of its strength, did not show significant effect on LEC proliferation and hence showed no direct effect on LECs (Fig. 2). The cord/tube formation assay serves as a model to understand the re-organization phase during lymphangiogenesis. Maximum tube length was significantly increased in negative (starvation medium) and positive (VEGF-A) control. In all other conditions, FM and both BoTA groups, tube length was significantly shorter. The average tube length was significantly increased in the VEGF-A medium. Non-significant increase was also observed in FM and BoTA medium. (Fig. 3a and b).
Ask a Question
Write your own review