
Immortalized Human Urothelial Cells
Cat.No.: CSC-I9089L
Species: Homo sapiens
Source: Urinary Bladder
Morphology: Epithelial-like
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT003 HT® Lenti-SV40T Immortalization Kit
CIK-HT013 HT® Lenti-hTERT Immortalization Kit
Researchers developed SV40-Immortalized Human Urothelial Cells (SV-HUC) by introducing the SV40 large T-antigen gene from Simian Virus 40 into human urothelial cells. Human urothelial cells form an epithelial layer that lines the bladder and other urinary organs like ureters and renal pelvis. The urothelial cells function as a protective barrier against external pathogens in the urinary system while also participating in urine formation and excretion processes.
SV-HUC enable researchers to study urinary system diseases and develop drugs while also analyzing gene functions. For instance, gene editing technologies utilize these cells to analyze the effects of oncogenic mutations on cellular behavior. Researchers conduct carcinogenicity and toxicity assessments of various compounds by exposing these cells to substances like p-phenylenediamine. Additionally, researchers use these cells to study how low-dose radiation affects human cells particularly concerning the development of bladder cancer.
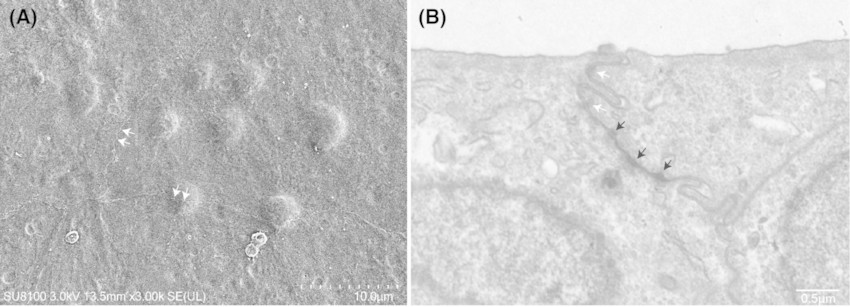 Fig. 1. Electron microscopy images of SV-40 immortalized human urothelial cells (SV-HUC-1 cells) (Luo HJ, Zhou H, et al., 2024).
Fig. 1. Electron microscopy images of SV-40 immortalized human urothelial cells (SV-HUC-1 cells) (Luo HJ, Zhou H, et al., 2024).
PPD Induced EMT in SV-HUC-1 Cells
Bladder cancer rates are increasing with PPD being a suspect carcinogen due to its presence in various consumer and industrial products. Epidemiological evidence links PPD exposure to multiple cancers. Liu's team studied PPD's potential in inducing EMT in SV-40 immortalized human urothelial cells through the ERK5/AP-1 pathway.
PPD was used on immortalized human urothelial cell line SV-40 (SV-HUC-1) to explore its relationship with bladder cancer. At concentrations between 0 and 5 μM, PPD showed no toxicity to cell viability (Fig. 1A). For further experiments, concentrations of 0.002, 0.01, and 0.05 μM were selected. EMT is marked by changes in cell shape, invasion, and migration. After PPD treatment, cells showed morphological changes, stretching into a spindle shape with pseudopodium formation (Fig. 1B). The wound healing and transwell assays demonstrated that the cells' migration and invasion abilities improved (Fig. 1C and D). Dose-dependent decreases in epithelial markers E-cadherin and ZO-1 were observed alongside increases in mesenchymal markers N-cadherin and vimentin through Western blotting results (Fig. 1E). qRT-PCR confirmed these mRNA expression changes (Fig. 1F). Immunofluorescence staining displayed cellular alterations caused by PPD treatment (Fig. 1G). The results demonstrate that PPD triggers EMT in SV-HUC-1 cells.
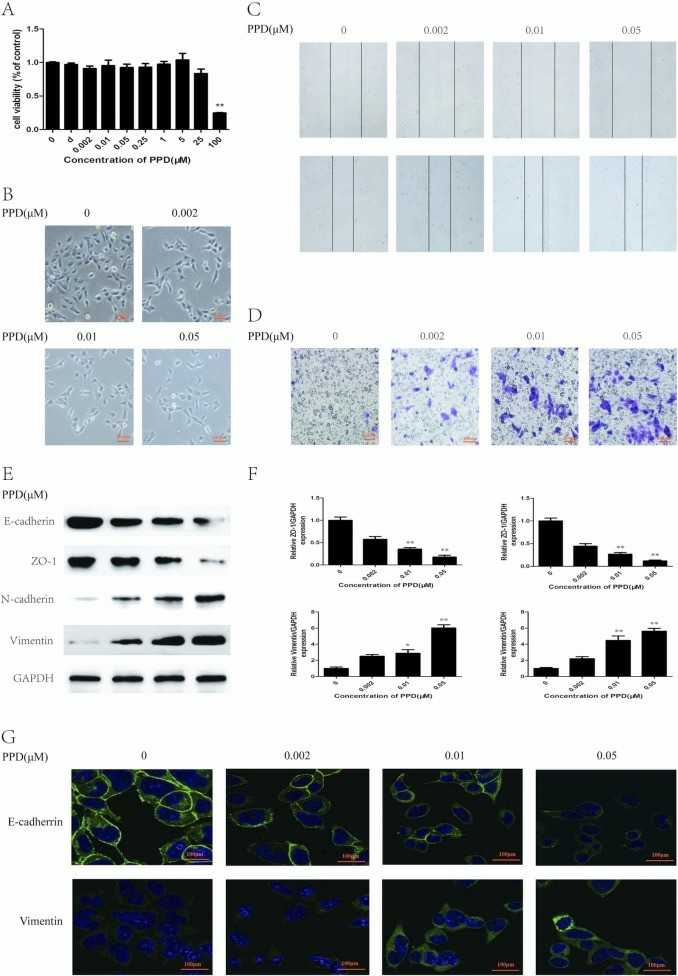 Fig. 1. PPD induces EMT in SV-HUC-1 cells (Liu ZQ, Zhao L, et al., 2022).
Fig. 1. PPD induces EMT in SV-HUC-1 cells (Liu ZQ, Zhao L, et al., 2022).
Hypoxia Down-Regulated the mRNA Expression of TJ- Proteins and Damaged TJ Structure in SV-HUC-1 Cells
Hypoxia plays an important role in the pathological process of bladder outlet obstruction. Previous research has mostly focused on the dysfunction of bladder smooth muscle cells, which are directly related to bladder contraction. Liu's team delved into the barrier function changes of the urothelial cells under exposure to hypoxia.
To assess hypoxia's impact on barrier-forming proteins, qPCR measured mRNA levels of AUM (UPIa, UPIb, UPII, UPIIIa) and TJ proteins (ZO-1, claudin1, occludin) in SV-40 immortalized human urothelial cells (SV-HUC-1). Short 2-hour oxygen deprivation didn't affect TJ protein expression, but 24-hour hypoxia significantly reduced ZO-1, claudin, and occludin mRNA (Fig. 2A-C). AUM protein levels remained unchanged (Fig. 2D-G). WB assay further detected the protein level of AUM proteins and TJ-associated proteins. Results revealed that 24-hour hypoxia and beyond substantially decreased TJ protein levels, with occludin showing the greatest reduction. AUM protein expression, including UPIa, UPIb, UPII, and UPIIIa, was largely stable. Immunofluorescence showed increased expression of TJ proteins. However, hypoxia reduced ZO-1, claudin-1, and occludin expression at cell junctions (Fig. 3).
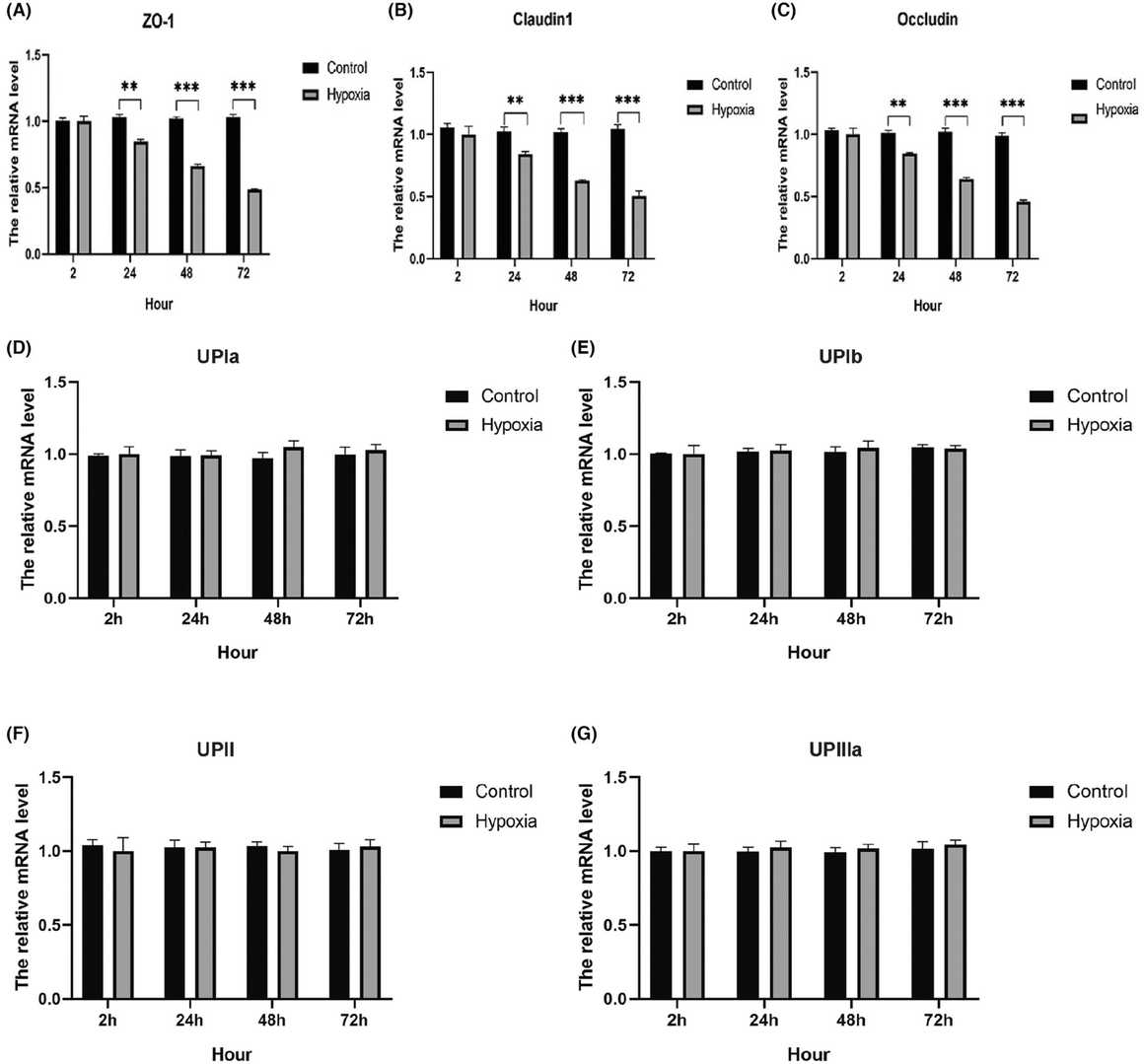 Fig. 2. Effect of hypoxia on the urothelial barrier-related gene expression (Liu HJ, Zhou H, et al., 2024).
Fig. 2. Effect of hypoxia on the urothelial barrier-related gene expression (Liu HJ, Zhou H, et al., 2024).
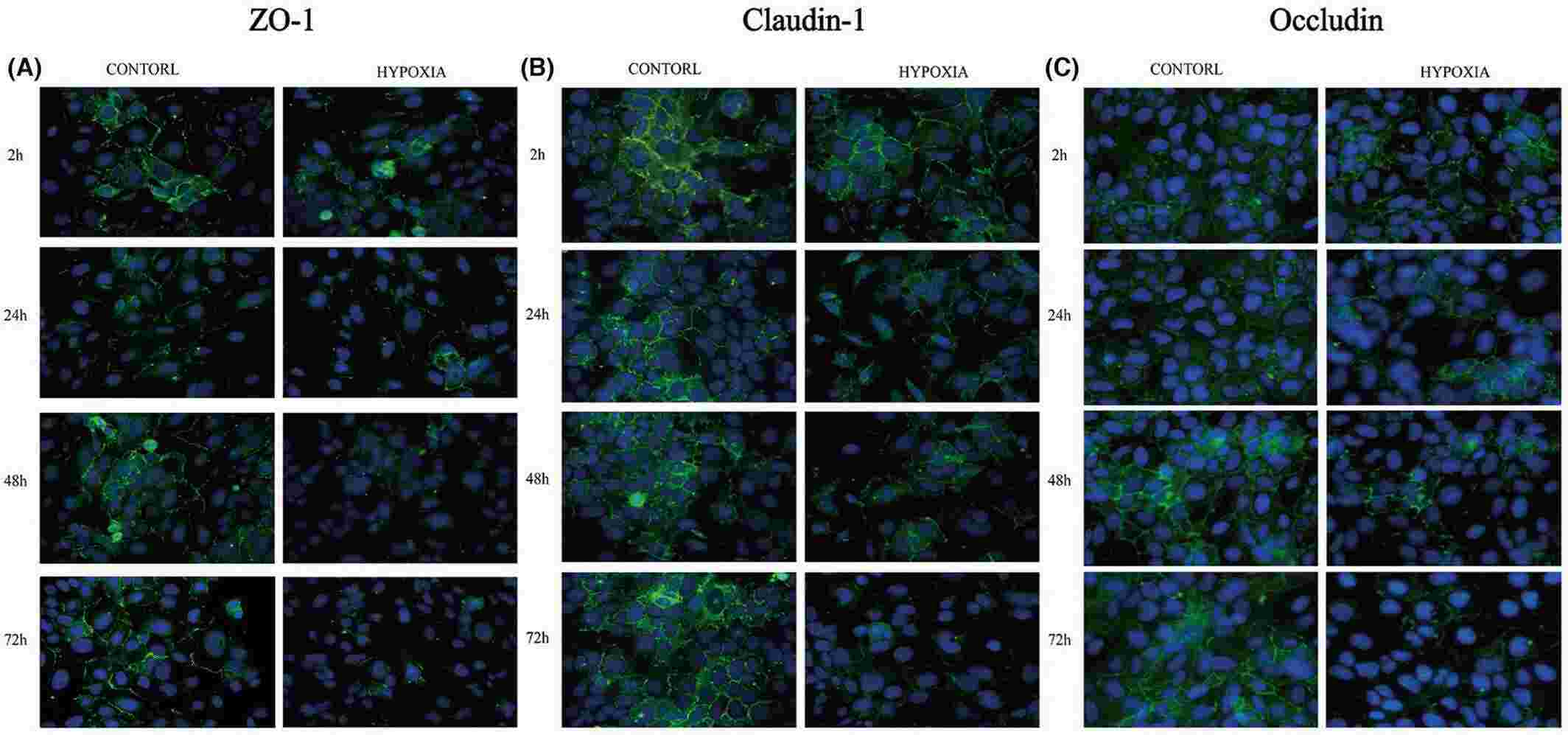 Fig. 3. Immunofluorescent staining of ZO-1, claudin-1 and occludin proteins under exposure to hypoxia (Liu HJ, Zhou H, et al., 2024).
Fig. 3. Immunofluorescent staining of ZO-1, claudin-1 and occludin proteins under exposure to hypoxia (Liu HJ, Zhou H, et al., 2024).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

