
Immortalized Human Umbilical Vein Endothelial Cells-hTERT
Cat.No.: CSC-I9007L
Species: homo sapiens
Source: Umbilical cord
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT013 HT® Lenti-hTERT Immortalization Kit
Human Umbilical Vein Endothelial Cells (HUVECs) are derived from human umbilical veins which serve as a popular choice for endothelial cell studies within vascular biology research. The umbilical veins serve as the main connection between the placenta and fetus because the endothelial cells in these veins demonstrate superior vascular functions and physiological properties which render them perfect for research on angiogenesis, thrombosis and vascular aging. The immortalization of these cells occurs due to hTERT gene overexpression following their isolation.
HUVECs immortalized with hTERT maintain normal endothelial cell morphology through their monolayer arrangement of polygonal cells containing typical Weibel-Palade bodies marked by vWF and PECAM-1 which demonstrates their characteristic endothelial biology. Cells under culture conditions maintain sustained growth in vitro beyond 33 passages while showing no signs of senescence or reduced replication competence. Moreover, these cells respond to angiogenic factors like VEGF and create new vascular networks which makes them valuable for researching angiogenesis mechanisms.
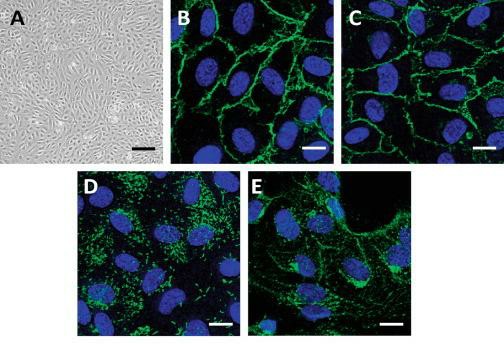 Fig. 1. Characterization of primary endothelial cells. (A) Confluent HUVEC monolayer grown on gelatin-coated plastic processed for microscopy. For immunofluorescence microscopy, confluent HUVECs were labeled with (B) anti-PECAM-1 (CD31), (C) anti-VE-cadherin, (D) anti-von Willebrand factor (VWF), and (E) anti-VEGFR2 antibodies as endothelial markers (green). The nucleus is labeled with DAPI (blue) (Fearnley G W, et al., 2014).
Fig. 1. Characterization of primary endothelial cells. (A) Confluent HUVEC monolayer grown on gelatin-coated plastic processed for microscopy. For immunofluorescence microscopy, confluent HUVECs were labeled with (B) anti-PECAM-1 (CD31), (C) anti-VE-cadherin, (D) anti-von Willebrand factor (VWF), and (E) anti-VEGFR2 antibodies as endothelial markers (green). The nucleus is labeled with DAPI (blue) (Fearnley G W, et al., 2014).
Inhibitory Effect of MK-0429 on Immortalized Human Umbilical Vein Endothelial Cells Growth, Migration, And Adhesion
Integrin αvβ3 is an essential molecule for tumor angiogenesis. Nakagawa et al. aimed to investigate the anti-tumor effect of MK-0429 on oral squamous cell carcinoma (OSCC) through its inhibitory effect on angiogenesis. They employed hTERT-immortalized human umbilical vein endothelial cells (HUEhT-1) and mouse oral cancer xenograft models to assess MK-0429's impact on cellular functions, angiogenesis, and tumor progression.
MK-0429 reduced HUEhT-1 cell proliferation in a dose-dependent fashion (Fig. 1a). The cytotoxic effect of MK-0429 was determined through LDH measurements in the culture medium. MK-0429 caused increased LDH levels in culture medium at high concentrations but did not show dose-related elevation (Fig. 1b). We conducted a wound healing assay to analyze how MK-0429 affects cell migration. The concentration of MK-0429 did not affect cell migration while a small dose of 1.0 μM MK-0429 suppressed it (Fig. 1c). Our study included an evaluation of how MK-0429 affected cellular adhesion to vitronectin, which served as an extracellular matrix material on culture plates. MK-0429 reduced the cell adhesion capacity of HUEhT-1 in a dose-dependent manner.
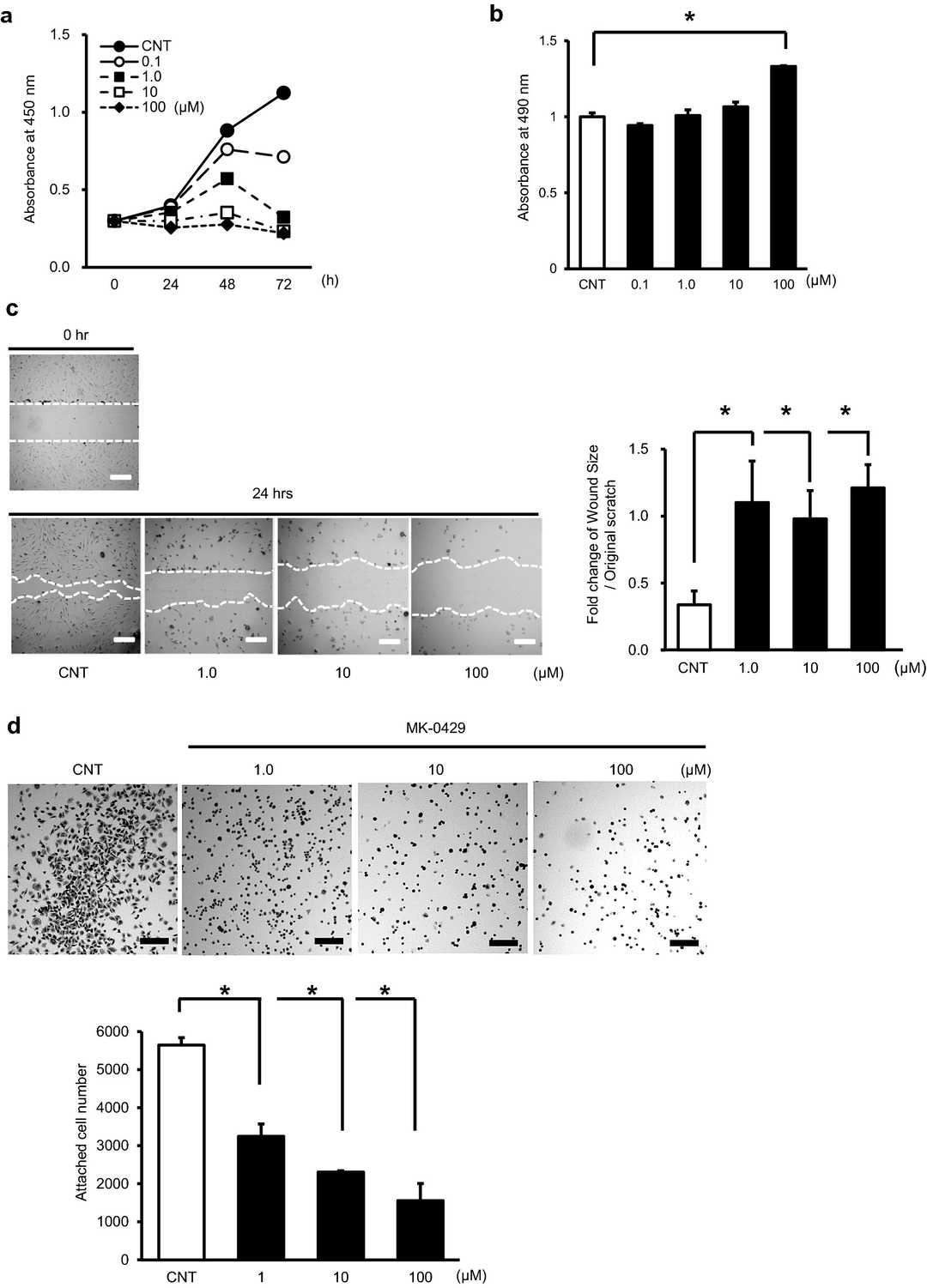 Fig. 1. Effects of MK-0429 on immortalized human umbilical vein endothelial cell, HUEhT-1 (Nakagawa T, Ohtaa K, et al., 2022).
Fig. 1. Effects of MK-0429 on immortalized human umbilical vein endothelial cell, HUEhT-1 (Nakagawa T, Ohtaa K, et al., 2022).
LEF Suppressed In Vitro Angiogenesis in Immortalized Human Umbilical Vein Endothelial Cells
Leflunomide (LEF) is a conventional synthetic disease-modifying antirheumatic drug and suppresses T-cell proliferation and activity by inhibiting pyrimidine synthesis using dihydroorotase dehydrogenase (DHODH); however, several studies have demonstrated that LEF possesses anticancer and antiangiogenic effects in some malignant tumors. Therefore, Niwata et al. investigated the anticancer and antiangiogenic effects of LEF on oral squamous cell carcinoma (OSCC).
In this study, a tube formation assay was used to examine LEF's impact on angiogenesis in vitro. Endothelial HUEhT-1 cells, derived from human umbilical vein cells and immortalized via hTERT electroporation, were utilized. These cells form vessels on extracellular matrix gels with angiogenic factors like VEGFs. As shown in Fig. 2a, the control group developed well-defined vascular structures, while the LEF-treated group showed disorganized, sparse structures dose-dependently. LEF significantly reduced vascular structures, junctions, meshes, segments, and total segment length at 10 and 100 μM doses compared to controls (Fig. 2b-e).
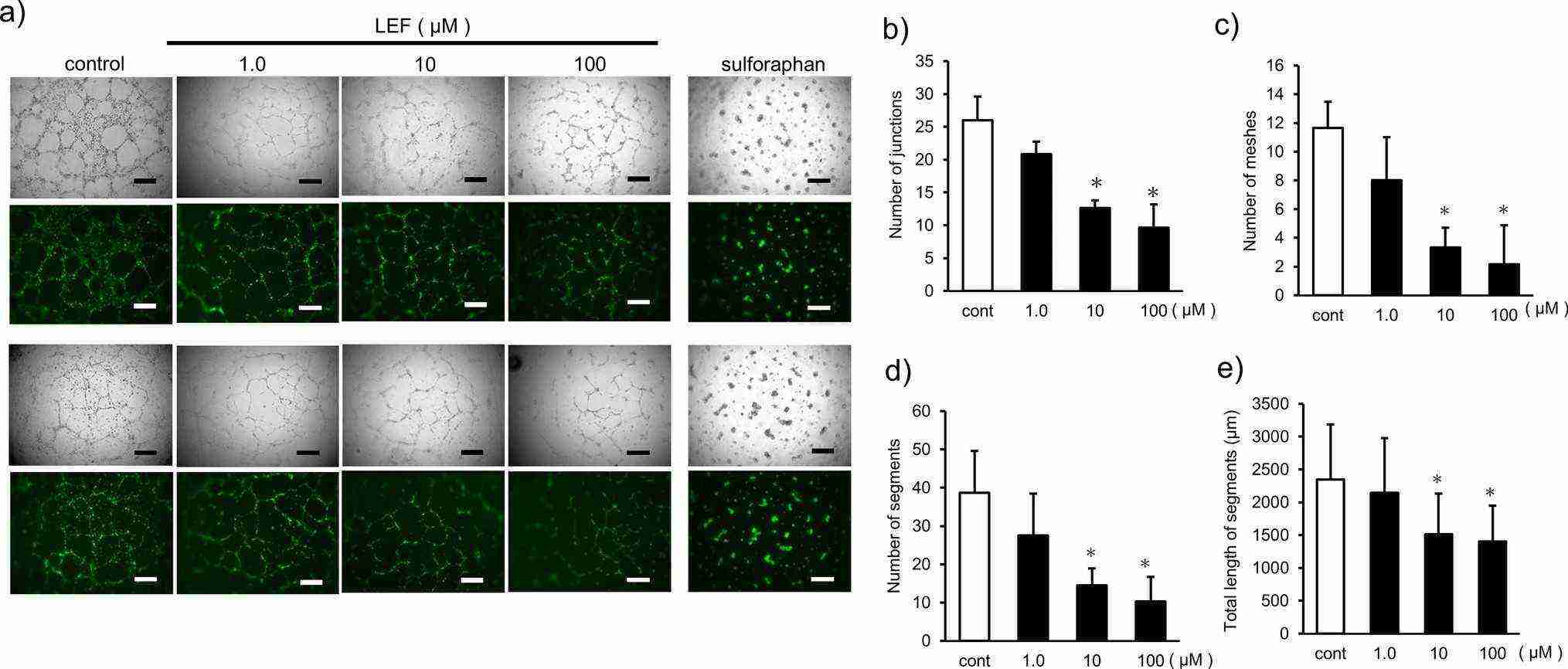 Fig. 2. Evaluation of the inhibitory effect of LEF on angiogenesis in vitro (Niwata C, Nakagawa T, et al., 2025).
Fig. 2. Evaluation of the inhibitory effect of LEF on angiogenesis in vitro (Niwata C, Nakagawa T, et al., 2025).
Telomerase activation is a common step in various cell immortalization methods. By expressing human telomerase re-verse transcriptase (hTERT), telomerase activity can be activated, telomere length can be maintained, chromosome telomere degradation can be prevented, and epithelial cell immortalization can be induced. The immortalized cells established by telomerase transfection can maintain the physiological characteristics of normal cells.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Professional
The sales staffs were very professional and provided me with many new insights for my experiments.
02 June 2021
Ease of use
After sales services
Value for money
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

