
Immortalized Human Podocytes
Cat.No.: CSC-I9325L
Species: Homo sapiens
Source: Urinary Tissue
Morphology: Cuboidal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT003 HT® Lenti-SV40T Immortalization Kit
2) Western blot preformed to determine SV40 T antigen expression
Creative Bioarray's immortalized human podocytes are derived from urine samples of healthy donors and have been transformed with the thermosensitive SV40 large T antigen (U19tsA58) and human telomerase (hTERT). Podocytes are a key component of the glomerular filtration barrier, located on the vascular side of the glomerulus, encasing the capillary loops. They interact with the basement membrane through their unique foot processes, filtering waste and excess water from the blood. Podocytes have complex morphological features; their cell bodies are large with multiple elongated primary processes that further branch into many tiny secondary processes. These foot processes are connected by slit diaphragms, allowing filtrate to pass through. Under culture conditions, these cells exhibit typical epithelial morphology, appearing polygonal or polyhedral.
Researchers use this cell line to investigate how podocytes react to hormonal and inflammatory stimuli to identify molecular pathways that lead to proteinuria. Also, this cell line serves as a testing platform in drug screening processes for evaluating pharmaceuticals designed to block inflammation while inhibiting fibrosis and protecting podocytes. By employing CRISPR-Cas9 technology to eliminate specific genes researchers examine how these genes affect podocyte function.
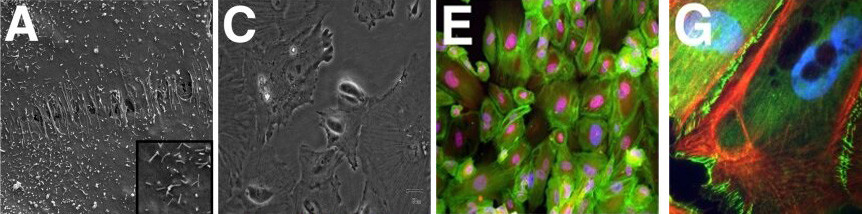 Fig. 1. Morphological of immortalized human podocytes, as shown by scanning electron microscopy (A, 100), light microscopy (C), immunofluorescence staining for β-actin (green) and nestin (red) (E), and immunofluorescence staining for F-actin (red) and ZO-1 (green) with a blue nuclear counterstain (DAPI) (G) (Herman M, Thomas M, et al., 2011).
Fig. 1. Morphological of immortalized human podocytes, as shown by scanning electron microscopy (A, 100), light microscopy (C), immunofluorescence staining for β-actin (green) and nestin (red) (E), and immunofluorescence staining for F-actin (red) and ZO-1 (green) with a blue nuclear counterstain (DAPI) (G) (Herman M, Thomas M, et al., 2011).
Characterization of Immortalized Human Podocytes Infected with Lentivirus as an In Vitro Model of Viral Infection-Associated Podocytopathy
Viral infections are linked to various kidney diseases through mechanisms such as systemic inflammation and direct infection of kidney cells. However, direct viral infection of podocytes and its resulting injury are poorly understood, necessitating effective experimental models.
Yu's team aimed to molecularly characterize immortalized human podocyte cell lines infected with lentivirus, using RNA-seq to assess the response and analyze pathways involved. Green fluorescence was visible three days post-infection (Fig. 1A and 1B). By day five, significant cell death was evident (Fig. 1C), indicating harmful effects of lentiviral infection. To investigate the mechanisms of this podocyte injury, they conducted RNA sequencing on infected and uninfected cells. Differential gene expression was visualized through heatmaps and volcano plots from EdgeR analyses (Fig. 2A and 2B). Bioinformatics further classified genes into GO terms like viral and immune system processes and KEGG pathways involving immune and infectious diseases. This revealed an immune response in the infected HPC cells. GO enrichment highlighted terms related to immune responses, such as MDA-5 and TLR7 signaling. KEGG enrichment included IL-17 signaling and cytokine interactions. TNF signaling and ferroptosis activations aligned with observed cell death (Fig. 1B). GSEA analysis highlighted significantly enriched terms.
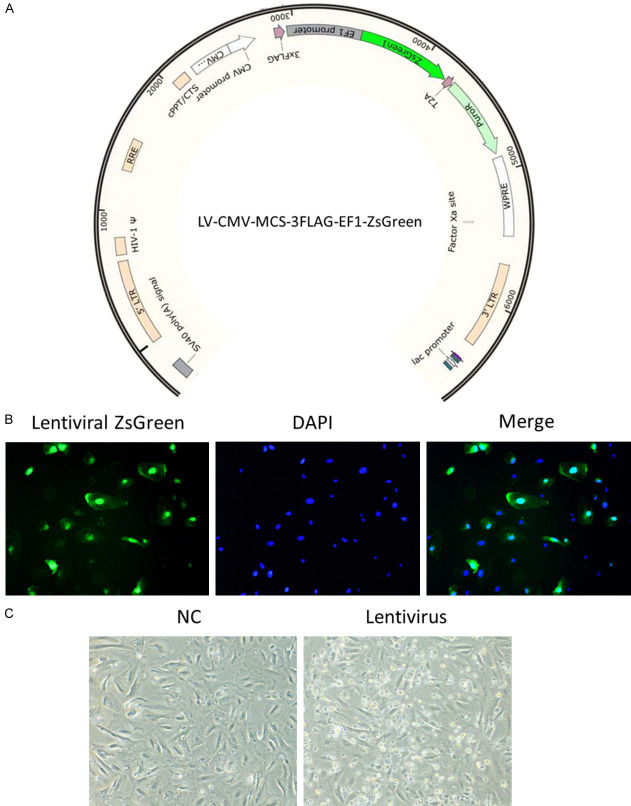 Fig. 1. Infection of lentivirus in HPCs induced cell death (Yu P, Jin X, et al., 2024).
Fig. 1. Infection of lentivirus in HPCs induced cell death (Yu P, Jin X, et al., 2024).
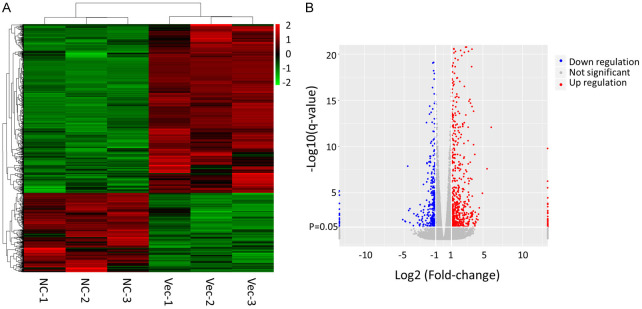 Fig. 2. Analysis of differentially expressed genes between control and retroviral infected HPC cells by EdgeR (Yu P, Jin X, et al., 2024).
Fig. 2. Analysis of differentially expressed genes between control and retroviral infected HPC cells by EdgeR (Yu P, Jin X, et al., 2024).
CXCL16 Regulates the Growth, Migration and Apoptosis of Human Podocytes
PNS damages podocytes and the glomerulus, leading to proteinuria and kidney disease. CXCL16, a chemokine family member, is linked to kidney diseases and oxidative stress-induced apoptosis, with ERK1/2 involved in cell signaling but understudied in PNS.
Previous studies have indicated that CXCL16 promotes mouse podocyte migration and regulates the progression of PNS; however, there are still areas of uncertainty. To clarify this, Chen's team examined the effects of CXCL16 after overexpression or knockdown on immortalized human podocytes (HPCS). HPCs were transfected with lentivirus to overexpress or knockdown CXCL16. Western blot analysis confirmed the overexpression or knockdown of CXCL16 (Fig. 3A and B). The overexpression of CXCL16 suppressed the growth of human podocytes and the knockdown of CXCL16 promoted the proliferation of podocytes (Fig. 3C and D). Consistent with this finding, CXCL16 overexpression promoted HPC apoptosis and the knockdown of CXCL16 suppressed the apoptosis of HPCs (Fig. 3E and F). In addition, trans-well assays were performed on HPCs stably transfected with CXCL16 or CXCL16 shRNA. The results indicated that CXCL16 overexpression promoted HPC migration (Fig. 3G), whereas CXCL16 knockdown significantly suppressed the migration of HPCs (Fig. 3H). Overall, these findings demonstrated that CXCL16 regulated the progress of PNS by inhibiting the growth and promoting the apoptosis of podocytes, which caused podocytes to lose the characteristics of normal cells and obtain a higher migration and movement ability.
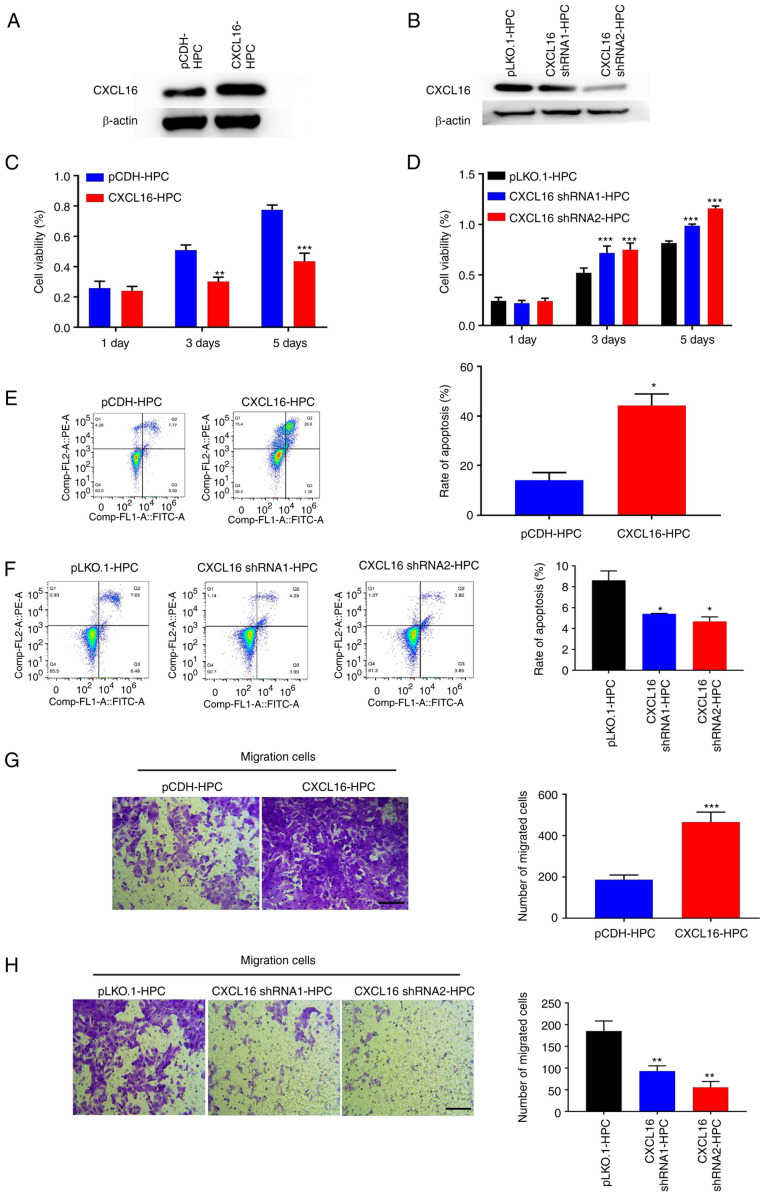 Fig. 3. CXCL16 regulates the growth of podocytes (Chen Y, Wang Z, et al., 2022).
Fig. 3. CXCL16 regulates the growth of podocytes (Chen Y, Wang Z, et al., 2022).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

