
Immortalized Human Hepatocytes-SV40
Cat.No.: CSC-I9016L
Species: homo sapiens
Source: Liver
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
- Documents
SuperCult® Immortalized Human Hepatocytes Culture Medium (Cat No.: CM-I9016L-C)
Note: Never can cells be kept at -20 °C.
CIK-HT003 HT? Lenti-SV40T Immortalization Kit
Free from contaminations (bacteria incl. mycoplasma, fungi, HIV, HAV, HBV, HCV, Parvo-B19) and cross-contaminations.
Human hepatocytes support numerous preclinical drug development disciplines, including in vitro evaluation of potential clinical effects of drugs and chemicals on the liver and, conversely, evaluation of possible hepatic effects of candidate compounds. Cryopreserved or plated human hepatocytes offer many advantages, but their usefulness is limited by unpredictable supply and significant individual variability in expression of drug-metabolizing enzymes and responses to toxicants. The availability of immortalized human hepatocytes with near-normal morphology and function greatly improves the efficiency, reproducibility, and predictive value of human hepatocyte studies.
Immortalized Human Hepatocytes are liver cells that have been genetically engineered to extend their functional lifespan in culture and maintain stable properties over short-term culture. These cells have shown promise for therapeutic applications, such as improving survival rates in preclinical models of acute liver failure when transplanted before the onset of severe damage.
Immortalized Human Hepatocytes do not proliferate indefinitely, but they are still of significant value in liver disease research and potential therapeutic applications due to their sustained functionality over short periods. Advances in cell engineering techniques, including telomerase activation via human telomerase reverse transcriptase (hTERT) and alterations in key regulatory pathways such as p16/RB or p53), have enhanced their potential for use in a variety of applications. Immortalized Human Hepatocytes are emerging as a key resource for the development of cell-based therapies for liver-related disorders.
MST3, STK25, and MST4 Play Overlapping but Nonredundant Roles in The Control of Hepatocellular Lipotoxicity
Metabolic dysfunction-associated steatotic liver disease has emerged as a leading global cause of chronic liver disease. Our recent translational investigations have shown that the STE20-type kinases comprising the GCKIII subfamily—MST3, STK25, and MST4—associate with hepatic lipid droplets and regulate ectopic fat storage in the liver; however, the mode of action of these proteins remains to be resolved. By comparing different combinations of the silencing of MST3, STK25, and/or MST4 in immortalized human hepatocytes, we found that their single knockdown results in a similar reduction in hepatocellular lipid content and metabolic stress, without any additive or synergistic effects observed when all three kinases are simultaneously depleted.
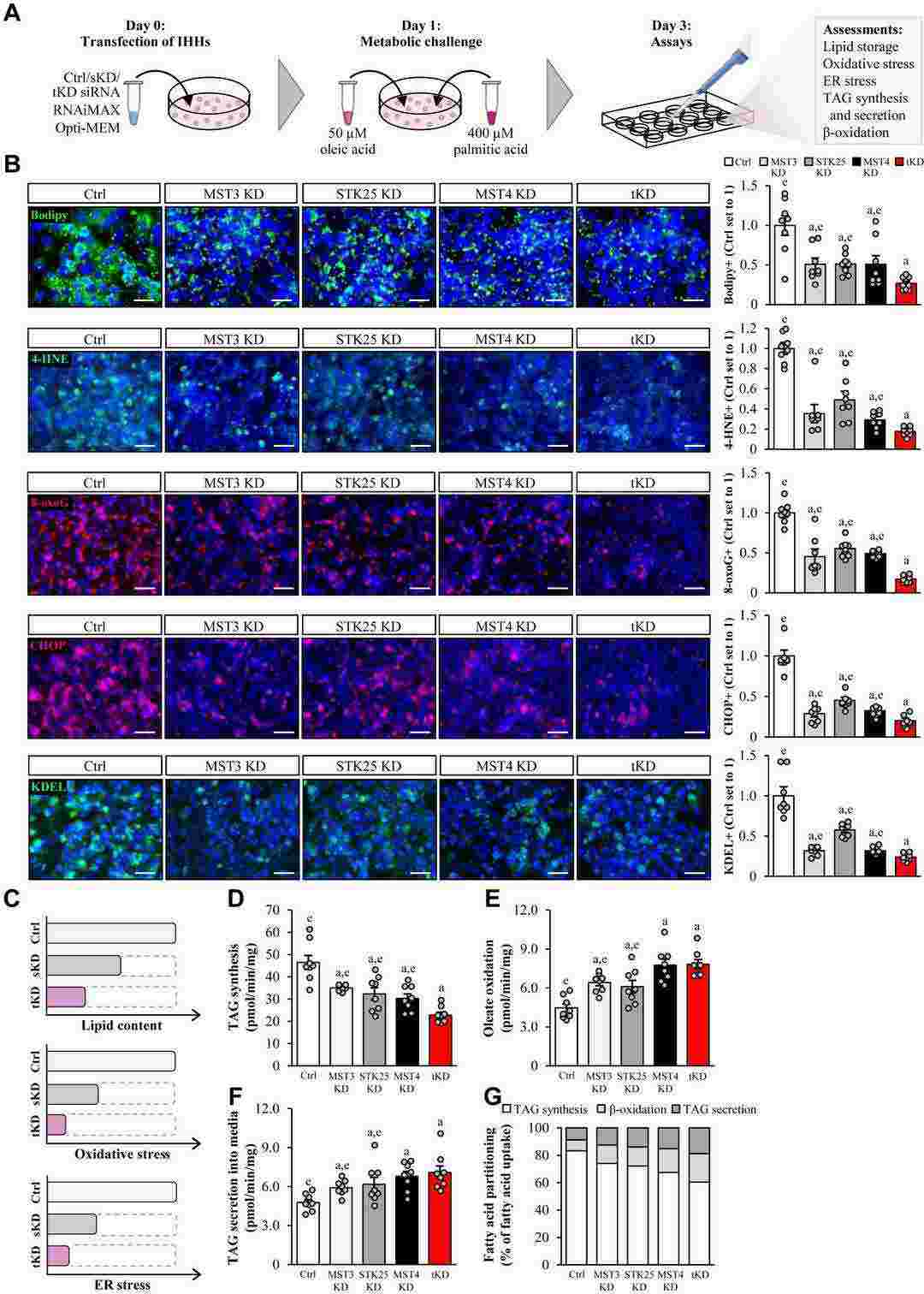 Fig. 1. Schematic illustration of the impact of single versus triple knockdown of the GCKIII kinases (MST3, STK25, and MST4) on hepatocellular lipotoxicity (Cansby, Emmelie, et al. 2024).
Fig. 1. Schematic illustration of the impact of single versus triple knockdown of the GCKIII kinases (MST3, STK25, and MST4) on hepatocellular lipotoxicity (Cansby, Emmelie, et al. 2024).
Effects on Cell Viability by Substances Migrating from the CMC-SA Edible Film to Food Simulants
Edible raw materials have gained attention as sustainable food packaging films, which are often considered a priori safe for human consumption. However, cytotoxicity issues may arise due to the incorporation of additives or modifications of film functionality during the manufacturing process. This study introduces an integrated methodology for the evaluation of potential migration of cytotoxic substances from materials used for the development of conventional and biodegradable food packaging. Carboxymethyl cellulose (CMC) and sodium alginate (SA) were tested as raw materials of an edible (CMC-SA) film, while a low-density-polyethylene (LDPE) film was tested as a conventional material. The potential migration of cytotoxic packaging substances into food simulants was evaluated using different human cells. Caco2 cells were used to simulate human intestine, whereas Huh7 and Immortalized Human Hepatocytes (IHH) cells simulated human liver. Cell viability assays and gene expression results indicated that substances migrating from the tested packaging materials neither produced cell cytotoxicity, nor induced oxidative stress to Caco2 cells.
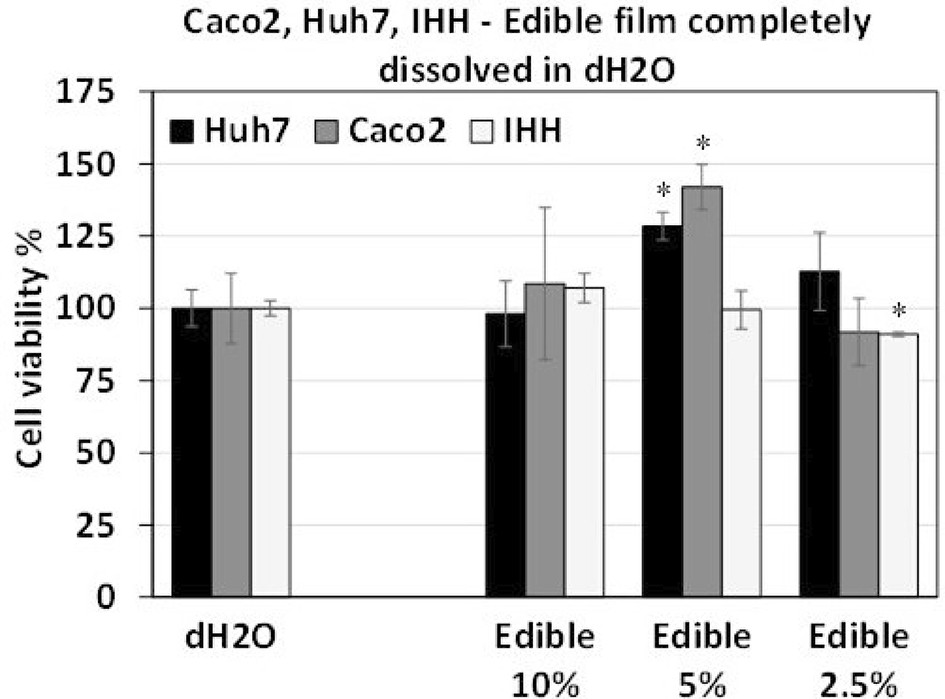 Fig. 2. Effect of the dH2O-dissolved CMC-SA edible film on survival of Huh7, Caco2 and IHH cells (Kalliampakou, Katerina I., et al. 2025).
Fig. 2. Effect of the dH2O-dissolved CMC-SA edible film on survival of Huh7, Caco2 and IHH cells (Kalliampakou, Katerina I., et al. 2025).
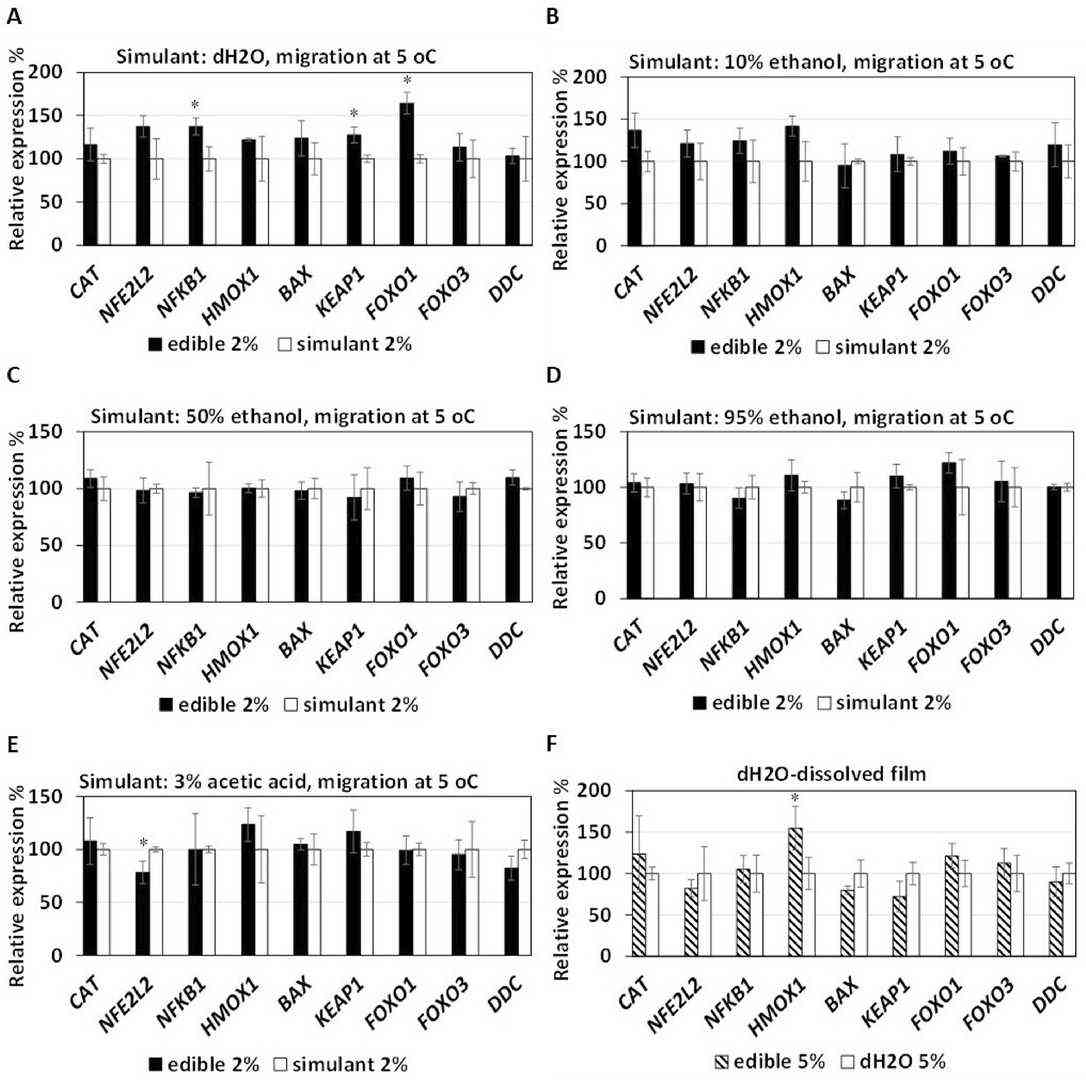 Fig. 3. Effect on the expression of oxidative stress-related genes by substances migrating from the CMC-SA edible film to food simulant solvents (Kalliampakou, Katerina I., et al. 2025).
Fig. 3. Effect on the expression of oxidative stress-related genes by substances migrating from the CMC-SA edible film to food simulant solvents (Kalliampakou, Katerina I., et al. 2025).
For optimal growth and maintenance, Immortalized Human Hepatocytes-SV40 should be cultured in a suitable medium. We recommend using:
SuperCult® Immortalized Human Hepatocytes Plating Medium (Cat No.: CM-I9016L-P)
SuperCult® Immortalized Human Hepatocytes Culture Medium (Cat No.: CM-I9016L-C)
Immortalized Human Hepatocytes-SV40 are widely used in various research applications, including:
Studies on liver metabolism and toxicity.
Drug metabolism and pharmacokinetics.
Research on mechanisms of liver disease and liver cancer.
Investigating liver-specific biomolecule storage and release.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells
