
Immortalized Human Hepatocytes-hTERT
Cat.No.: CSC-I9017L
Species: Homo sapiens
Source: Liver
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT013 HT® Lenti-hTERT Immortalization Kit
The "Immortalized Human Hepatocytes-hTERT" cell line is derived from human liver tissue. The liver serves as the main metabolic organ in the body because it performs essential physiological functions such as detoxification, metabolism, and hormonal regulation. By introducing the human telomerase reverse transcriptase (hTERT) gene into human liver cells researchers have created a cell line that bypasses the natural proliferative limits of normal hepatocytes to achieve immortality. This immortalized cell line demonstrates many primary hepatocytes functions such as drug metabolism maintenance, bile secretion, and glycogen synthesis. It produces multiple liver-specific proteins which feature drug-metabolizing enzymes such as CYP2C9 and CYP3A4. The unique characteristics of this cell line make it a perfect model system for research on liver diseases as well as drug metabolism and liver function replacement technologies.
The cell line serves as a primary research tool to investigate liver-associated pathological and metabolic mechanisms such as liver fibrosis and cirrhosis along with non-alcoholic fatty liver disease and beyond. Co-culturing with hepatic stellate cells enables researchers to observe liver fibrosis progression and understand the molecular basis of disease development. Drug screening with the cell line enables researchers to assess new drug hepatotoxicity and metabolic impacts which helps speed up the drug development process. Additionally, it presents a model system for regenerative liver medicine which supports the creation of new liver function replacement therapies. Researchers can use these cell lines to test gene editing methods aimed at discovering treatments for genetic liver conditions.
Overexpression of Centenarian Variants of SIRT6 does not Affect Hepatocyte Insulin Sensitivity
NAFLD, impacting 30% of the global adult population, escalates into more severe conditions like cirrhosis and hepatocellular carcinoma with no approved specific treatments. The discovery of genetic variants of SIRT6, a protein regulating aging and cellular stress, offers insights into novel therapeutic avenues. Frohlich et al. employed in vitro hepatic models using immortalized human hepatocytes (IHH) and hepatic stellate cells to investigate the metabolic and secretomic profiles affected by centenarian-associated SIRT6 mutations.
Figure 1A shows the far-red fluorescence protein Katushka2S signal in the LV cassette, either alone or with SIRT6 versions (WT, N308K, or N308K/A313S), demonstrating successful infection. No signal was detected in IHH control cells. Western blot analysis (Fig. 1B and C) showed a significant increase in SIRT6 levels in cells transfected with LV-SIRT6 compared to empty or CTL cells. SIRT6 represses gene transcription by deacetylating H3K9 and H3K56. Thus, in SIRT6 overexpression (OE) groups, acetylated histone H3K9 levels were significantly reduced, and H3K56Ac levels showed a decreasing trend (Fig. 1B and C), indicating increased SIRT6 expression and deacetylase activity. Given the close association of insulin receptor substrate PI3K (phosphoinositide 3-kinase) and AKT signaling pathways with metabolic disorders and insulin resistance, they tested by immunoblotting whether SIRT6 overexpression and longevity variants affected insulin sensitivity/PI3K/AKT pathway activation in IHH cells. After serum and glucose starvation, cells were stimulated with human insulin solution (100 nM) for 30 min. pAKT (Ser473) levels were measured, revealing no significant differences among conditions, with or without insulin (Fig. 2A and B).
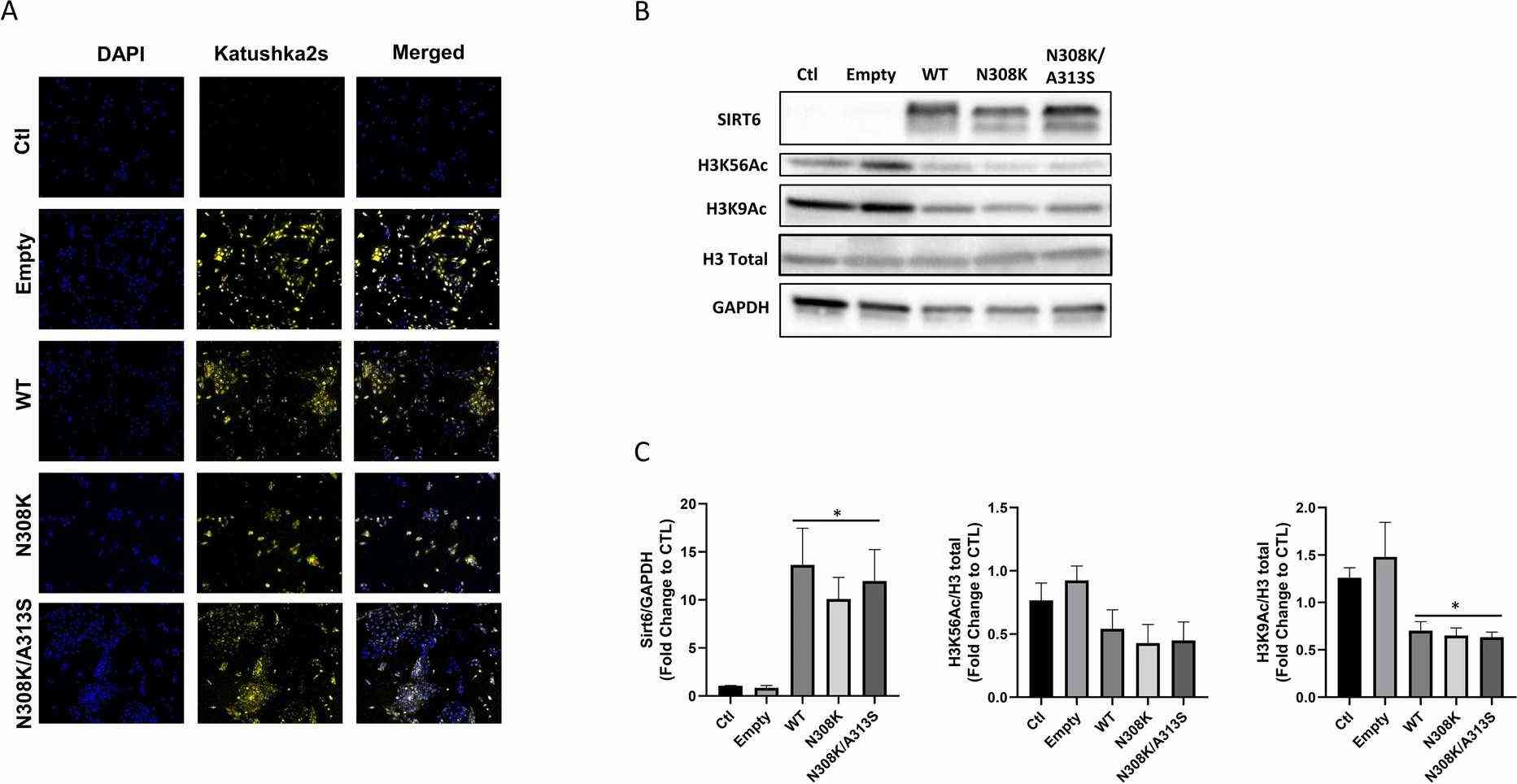 Fig. 1. Model of stable transfected IHH cells overexpressing SIRT6 allele variants (Frohlich J, Raffaele M, et al., 2022).
Fig. 1. Model of stable transfected IHH cells overexpressing SIRT6 allele variants (Frohlich J, Raffaele M, et al., 2022).
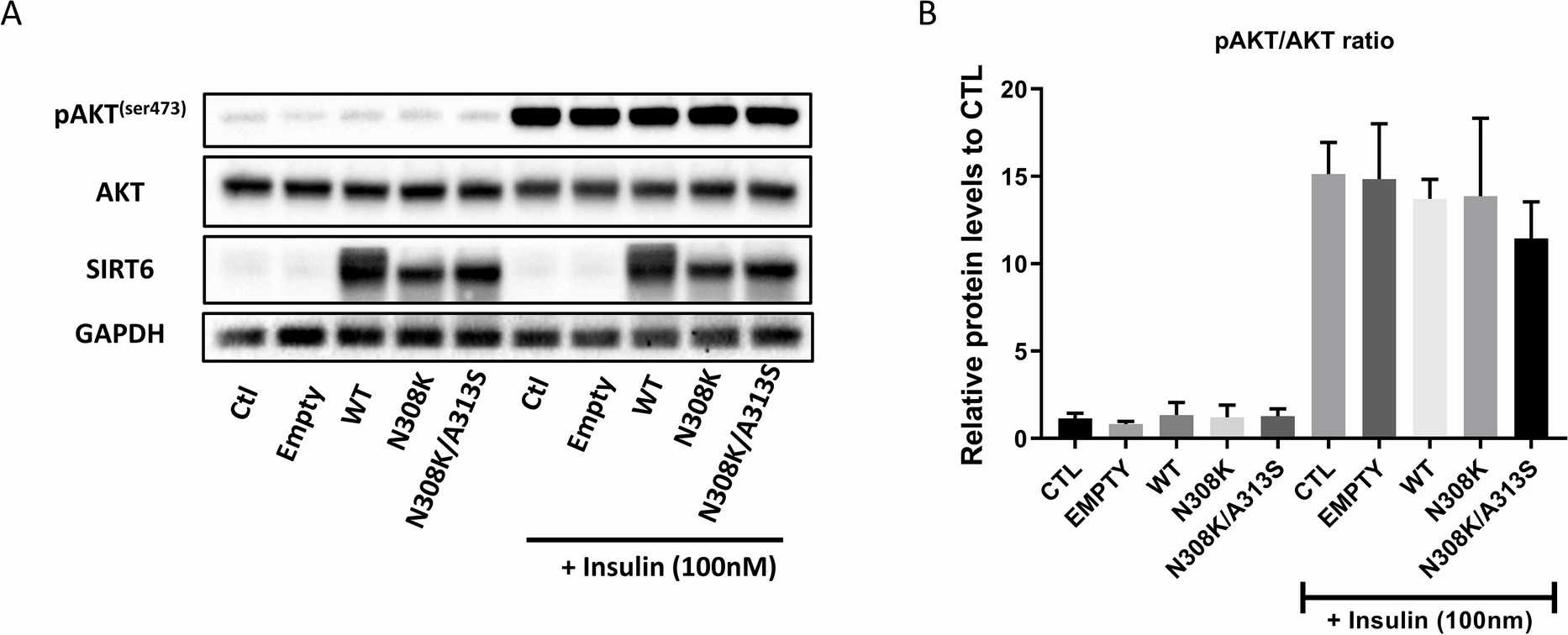 Fig. 2. SIRT6 overexpression did not affect pAKT/AKT ratio in IHH (Frohlich J, Raffaele M, et al., 2022).
Fig. 2. SIRT6 overexpression did not affect pAKT/AKT ratio in IHH (Frohlich J, Raffaele M, et al., 2022).
Prolonged Exposure of Human Hepatocytes to Hyperinsulinemia Enhances Cell Senescence
NAFLD affects the liver through multiple conditions ranging from simple steatosis to advanced hepatocellular carcinoma without available drug treatments. The aging process features cellular senescence which shows connections to various metabolic diseases. Cells experience senescence when exposed to hyperinsulinemia which is related to obesity and insulin resistance. Baboota et al. explored chronic hyperinsulinemia's direct role in inducing senescence in human hepatocytes.
To examine prolonged hyperinsulinemia's effect on hepatic senescence, we treated immortalized human hepatocytes (IHH) with insulin (20 and 100 nM) for 6, 24, or 72 hours. Insulin consistently increased protein senescence markers MDM2, p53, and p21 (Fig. 3A). Similarly, IGF1, activating a related pathway, elevated these markers' levels (Fig. 3A). Immunostaining confirmed the p53 increase from insulin and IGF1 (Fig. 3B). Insulin and IGF1 led to γH2AX puncta, indicating senescence-related DNA damage (Fig. 3B), and raised p16 and SASP markers like IL18, MMP3, IL32, and IL8 mRNA levels. These results show that extended high insulin and IGF1 exposure boosts senescence marker expression in human hepatocytes.
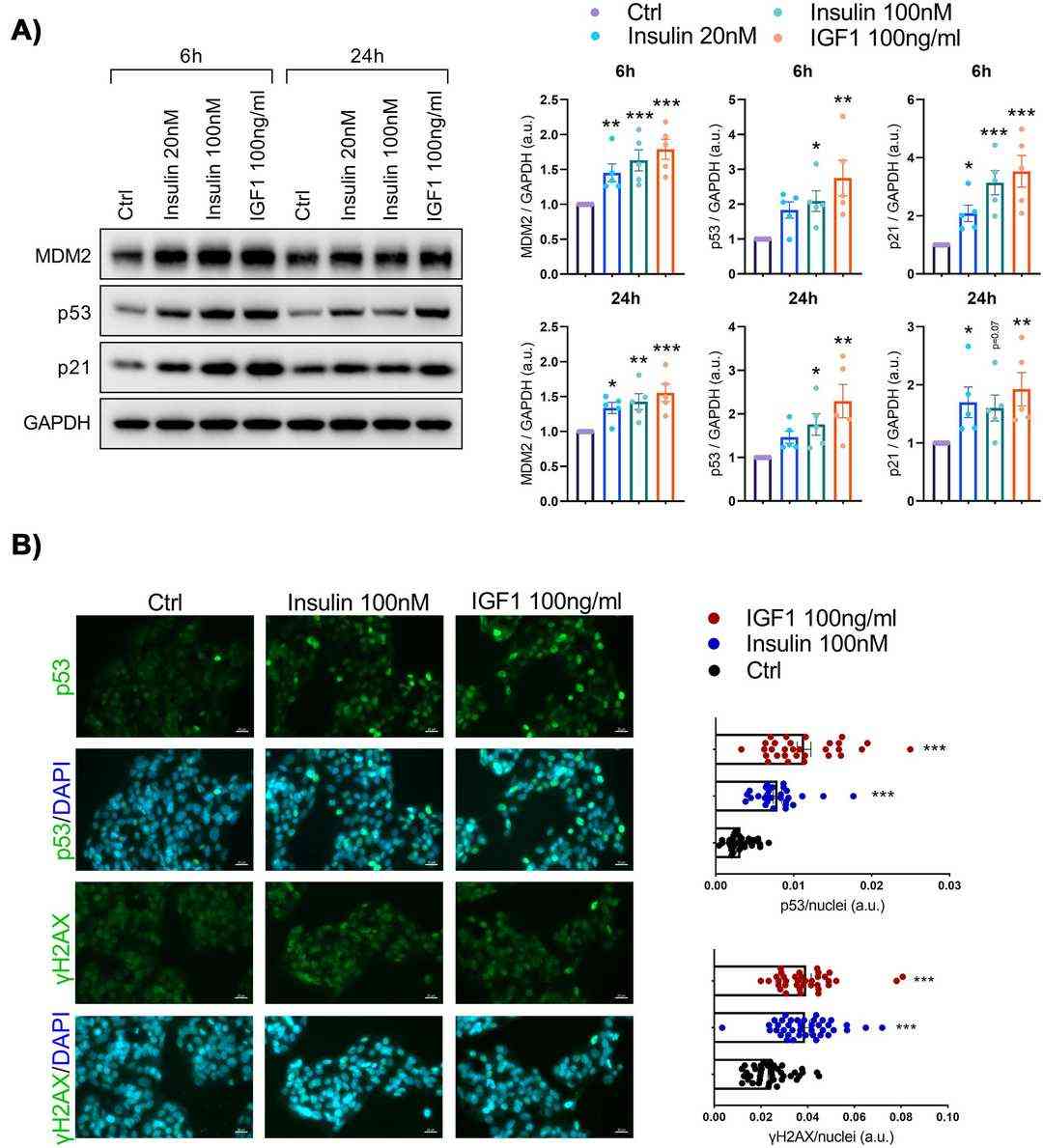 Fig. 3. Senescence markers are increased in human hepatocytes following insulin or insulin-like growth factor 1 (IGF1) exposure (Baboota R K, Spinelli R, et al., 2022).
Fig. 3. Senescence markers are increased in human hepatocytes following insulin or insulin-like growth factor 1 (IGF1) exposure (Baboota R K, Spinelli R, et al., 2022).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells
