
Immortalized Human Gingival Keratinocytes (Gie-No3B11)
Cat.No.: CSC-I9236L
Species: homo sapiens
Source: Buccal Gingiva
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT003 HT® Lenti-SV40T Immortalization Kit
Primary gingival keratinocytes extracted from human oral gingival tissue underwent immortalization to create the Gie-No3B11 cell line. The oral mucosa contains gingival tissue which is located between the teeth and the alveolar bone. Gingival tissue acts as a protective barrier that keeps periodontal tissue safe from bacteria and harmful substances. The Gie-No3B11 cell line displays morphological characteristics which are identical to those seen in primary gingival keratinocytes. The cells generate cytokeratins such as cytokeratin 2 alongside cytokeratin 17 and cytokeratin 19 and exhibit standard polygonal or irregular keratinocyte shapes. Furthermore, these cells show the capability to develop dense multilayer structures.
Scientists use the Gie-No3B11 cell line as a standard model to study both periodontal disease development and oral mucosal cancer progression. They can also be co-cultured with other cells (such as fibroblasts) to construct three-dimensional tissue equivalents, which are used to study tissue repair and regeneration processes.
Metabolic Activity of Gingival Epithelial Cells in Response to Core-Multishell Nanocarriers
Drug delivery systems target the oral mucosa because it provides easy access and avoids hepatic first-pass metabolism. The bioavailability of drugs used in local therapy for oral diseases decreases because of the mucosal barrier and the diluting effects of saliva and crevicular fluid. To address this, Jager et al. characterized core-multishell (CMS) nanocarriers for their potential use in oral mucosal drug delivery.
To evaluate potential cytotoxic effects of the CMS nanocarrier, we performed MTT assays to monitor the metabolic activity (cell survival) of cells after CNS nanocarrier application (Fig. 1). They used the immortalized gingiva keratinocytes Gie-No3B11 due to an intended topical use of the particles at the oral mucosa. The application of the unloaded CMS nanocarrier did not affect viability of the cells at concentrations ranging from 1 to 50 μg/mL after 24 hours. The higher concentrations 100 and 500 μg/mL slightly decreased the metabolic activity when compared to control epithelial cells (Fig. 1A). After 48 hours, CMS nanocarriers did not influence epithelial cell survival at any concentration tested (Fig. 1B). After 72 hours, the metabolic activity was unaffected at CMS concentrations between 1 and 100 μg/mL, and a reduced metabolic activity was found for the highest concentration (500 μg/mL) applied compared to control cells (Fig. 1C).
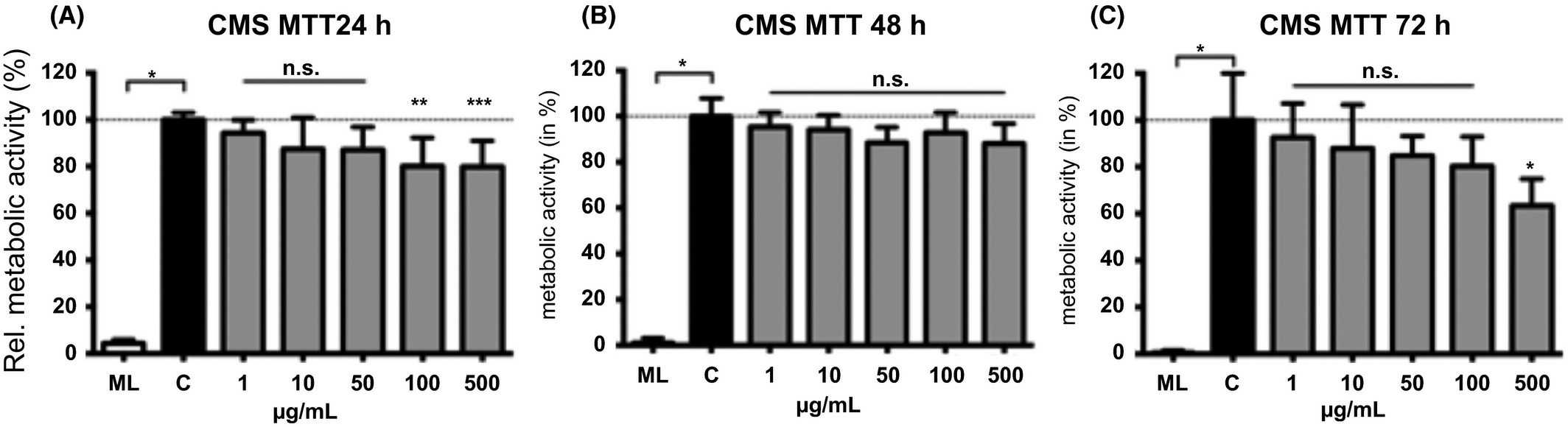 Fig. 1. Metabolic activity in gingival epithelial cells was monitored when exposed to different concentrations the CMS nanocarrier for 24 (A), 48 (B) or 72 hours (C) using the MTT assay (Jager J, Obst K, et al., 2018).
Fig. 1. Metabolic activity in gingival epithelial cells was monitored when exposed to different concentrations the CMS nanocarrier for 24 (A), 48 (B) or 72 hours (C) using the MTT assay (Jager J, Obst K, et al., 2018).
Glycation of Host Proteins Increases Pathogenic Potential of Porphyromonas gingivalis
The connection between periodontitis and diabetes exists because diabetes raises periodontitis risk through increased inflammatory responses and protein glycation. The non-enzymatic process of glucose binding to proteins known as glycation occurs frequently in diabetic individuals and can modify protein functions. Śmiga et al. aim to determine if glycated proteins enhance the growth and pathogenic potential of Porphyromonas gingivalis, a key periodontal pathogen.
They found that glycation increases the ability of P. gingivalis to acquire heme from hemoglobin, mostly due to heme sequestration by the HmuY hemophore-like protein. Based on their results, they hypothesize that differences in hemoglobin degradation and heme release between glycated and un-glycated hemoglobin may not significantly enhance P. gingivalis growth in laboratory liquid culture media. P. gingivalis-mediated diseases involve the bacterium's ability to infect host cells and evade immune defenses. We examined how P. gingivalis infected keratinocytes cultured with hemoglobin as the sole heme source. Glycated hemoglobin slightly increased P. gingivalis infection ability, mainly through enhanced bacterial attachment rather than invasion (Fig. 2). The evidence indicates that glycation could permit P. gingivalis survival and deeper penetration into the epithelium through intercellular pathways. Statistical analysis revealed no significant differences between cultures containing glycated hemoglobin and those containing un-glycated hemoglobin. The simple keratinocyte culture model used for this test likely fails to completely simulate real-life in vivo conditions. Research with a three-dimensional gingival model featuring collagen microfibers revealed comparable results.
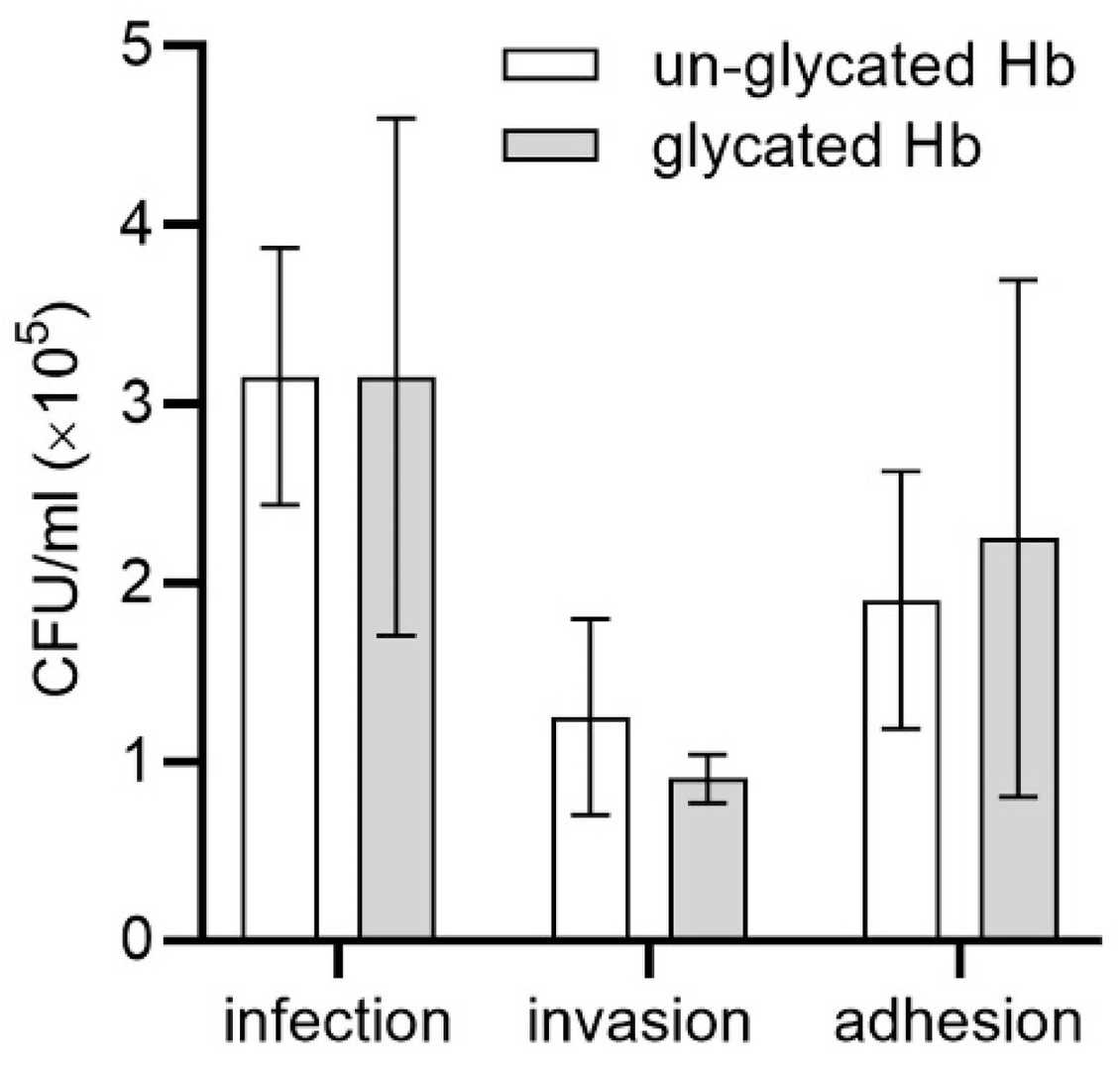 Fig. 2. Comparison of HmuY-Fe(III)heme complex formation from hemoglobin (Śmiga M, Smalley JW, et al., 2021).
Fig. 2. Comparison of HmuY-Fe(III)heme complex formation from hemoglobin (Śmiga M, Smalley JW, et al., 2021).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

