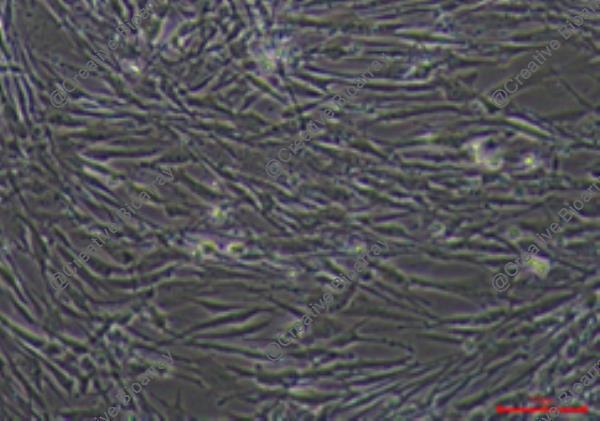
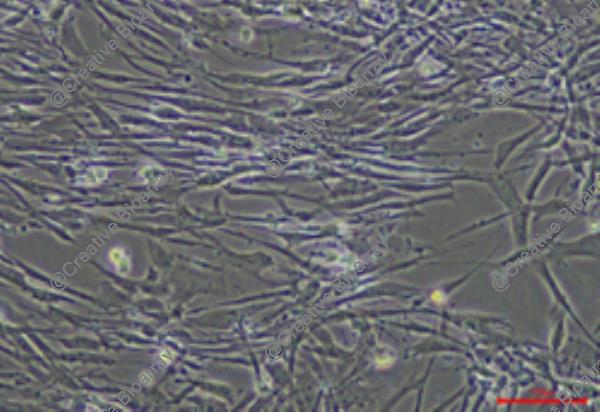
Immortalized Human Endometrial Epithelial Cells-SV40
Cat.No.: CSC-I2078Z
Species: homo sapiens
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
- Documents
Note: Never can cells be kept at -20°C.
The "Immortalized Human Endometrial Epithelial Cells-SV40" cell line is an in vitro cultured immortal line by incorporating the SV40 large T-antigen gene. The cell line originates from epithelial cells of the human endometrium which forms a crucial part of the female reproductive system located on the uterine cavity's inner wall. The endometrium provides essential nutritional support for embryo implantation while undergoing growth and shedding phases during each menstrual cycle. Endometrial epithelial cells maintain uterine conditions and hormonal equilibrium as they lead embryonic development.
This immortalized cell maintains their inherent functional properties including their responses to estrogen and progesterone hormones. Researchers can track endometrial functions by analyzing gene expression patterns in cells that express placental protein (PLAP), fibronectin (FN), and tissue plasminogen activator (t-PA). The cells mimic endometrial responses to ovarian steroid hormones by producing IGF-1 when stimulated by estradiol and progesterone. Scientists can use this cell line to study how endometrial epithelial cells function in hormone regulation as well as their growth and differentiation activities. Additionally, the connection between endometrial epithelial cells and both endometriosis and endometrial cancer makes this cell line a useful research model for studying disease pathogenesis.
Oleuropein Selectively Inhibited ERβ Activity but not ERα Activity
Endometriosis, characterized by ectopic endometrial tissue growth, is linked to inflammation and high estrogen levels. Current hormone-based treatments often have adverse effects. Park et al. targets estrogen receptor β (ERβ) for alternative solutions. Researchers employed cell-based assays and mouse models to screen natural products, identifying oleuropein as an ERβ inhibitor. How does oleuropein inhibit ERβ activity? To find out, the location of ERβ in immortalized human endometrial epithelial cells overexpressing ERβ (IHEECs:ERβ) was checked after 24 h of treatment with vehicle, estradiol (10 nM), oleuropein (10 nM) or estradiol plus oleuropein. Without estradiol, most ERβ was in the cytoplasm (Fig. 1d). Estradiol moved ERβ to the nucleus (Fig. 1e), but oleuropein did not enhance this (Fig. 1f). Moreover, oleuropein blocked estradiol-induced ERβ nuclear localization (Fig. 1g).
To find natural products that lower ERβ protein levels, a luciferase-ERβ fusion protein expression vector was made. The assay showed that oleuropein did not affect luciferase activity (Fig. 1h), meaning it did not change ERβ protein levels. This was confirmed using human endometriotic stromal cells, which have high ERβ levels. These cells showed no change in ERβ protein levels after oleuropein treatment (Fig. 1i). In summary, oleuropein inhibits ERβ activity without degrading ERβ protein.
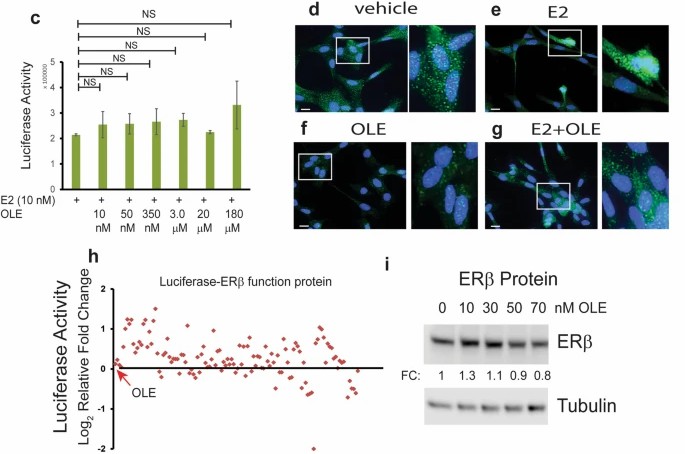 Fig. 1. Inhibition of ERβ activity by oleuropein a Natural product screening for ERβ-selective inhibitors (Park Y, Cho Y J, et al., 2022).
Fig. 1. Inhibition of ERβ activity by oleuropein a Natural product screening for ERβ-selective inhibitors (Park Y, Cho Y J, et al., 2022).
The ERβ/HDAC8 Axis Leads to the Reduction in NMI Expression in Endometrial Stromal Cells
Endometriosis is a condition characterized by the growth of endometrial tissue outside the uterus, affecting 10% of women of reproductive age. The estrogen-dependent nature of this condition has made hormonal therapy a common treatment, though it presents unwanted side effects.
Using techniques like gene knockdown in immortalized human endometrial stromal cells and observing ectopic lesion growth in mouse models, Park et al. found that NMI levels are reduced in stromal cells of human endometriotic lesions. However, no significant difference was found in NMI levels between ERβ-overexpressing and wild-type (WT) eutopic endometrium (Fig. 2A). they used Myc-tagged ERβ-overexpressing immortalized human endometrial epithelial cells (IHEECs) and stromal cells (IHESCs) to validate our finding. MYC-tagged ERβ was expressed robustly in both cells, as confirmed by Western blot with an MYC-antibody (Fig. 2B). ERβ overexpression significantly reduced NMI expression in IHESCs, but not in IHEECs, aligning with stromal cell-specific NMI reductions in endometriotic lesions (Fig. 2E), indicating an inverse correlation between ERβ and NMI in lesions. They thus explored how ERβ reduces NMI in lesions, investigating ERβ's interaction with NMI's promoter in lesions via lesion-specific ERβ ChIP-seq. This analysis showed ERβ binding to the NMI promoter in lesions (Fig. 2C). Both normal and endometriotic tissues show ERβ binding to the NMI promoter, but higher ERβ and local estrogen levels in lesions enhance ERβ's active binding under high estrogen, showing ERβ-mediated NMI suppression in lesions but not in normal endometrium.
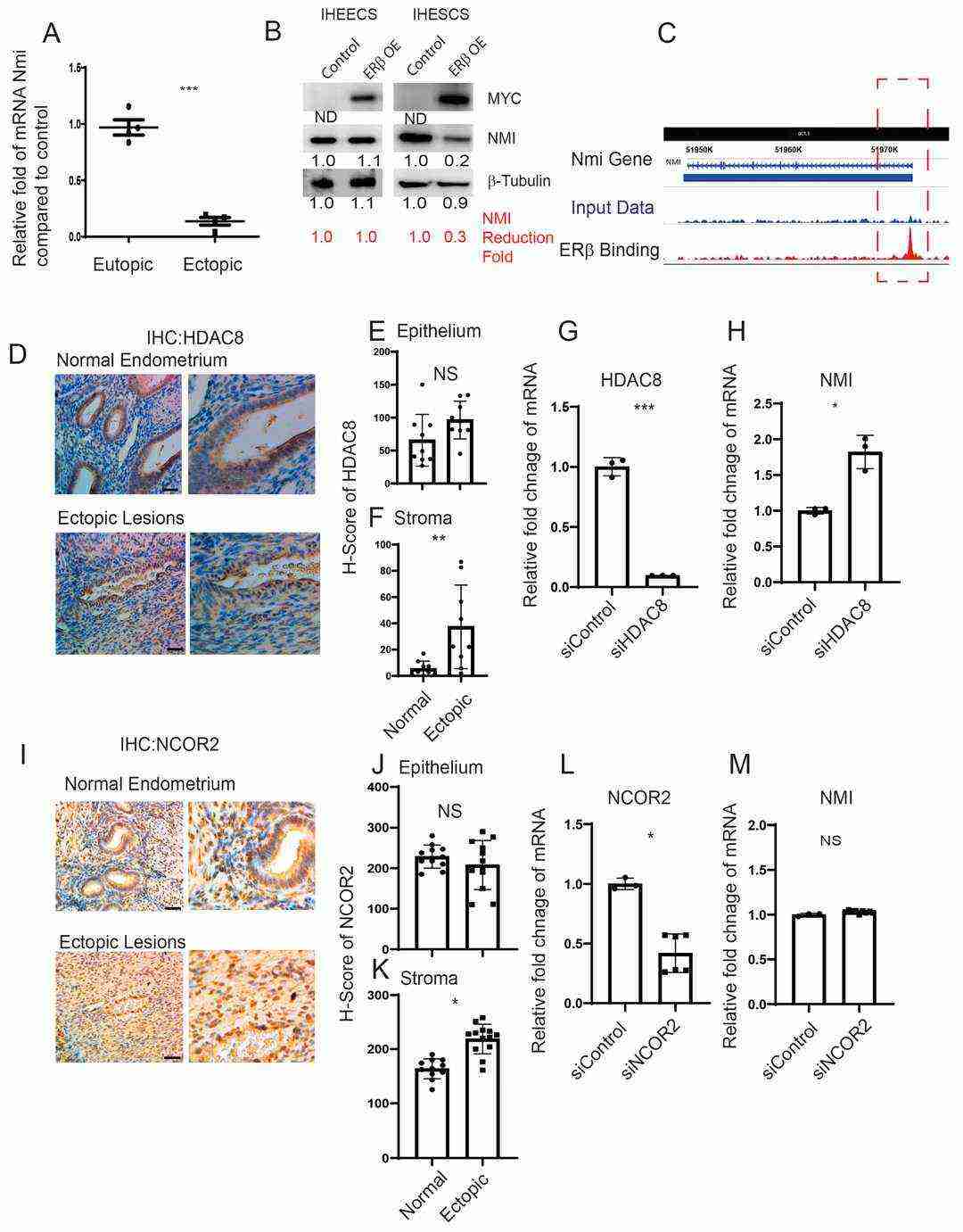 Fig. 2. Modulation of NMI expression via the ERβ/HDAC8 axis in endometrial stromal cells (Park Y, Guan X, et al., 2024).
Fig. 2. Modulation of NMI expression via the ERβ/HDAC8 axis in endometrial stromal cells (Park Y, Guan X, et al., 2024).
Yes, we offer a variety of custom services, including:
Genetic modifications (e.g., reporter gene insertion, gene knock-in/knockout).
High-volume production.
Custom assay development and optimization.
Tailored media formulation.
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells