Porcine Hepatocytes
Cat.No.: CSC-C4926L
Species: Pig
Source: Liver
Cell Type: Hepatocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
Porcine hepatocytes are one of the most popular cell lines used in bioartificial liver (BAL) systems. A BAL is an extracorporeal support system that is used to replace liver function in acute liver failure (ALF) patients until their liver recovers or they receive a liver transplant. Porcine hepatocytes are preferred over other cell lines due to their functional similarity to human hepatocytes and the easy availability of pig liver supplies.
Porcine hepatocytes are polygonal cells with a diameter of 20–30 μm. These cells display prominent nucleoli and double nuclei in their large nuclear regions. The cytoplasm is highly rich in mitochondria (1000–2000 per cell). The hepatocyte surface is covered with microvilli that increase the absorptive area and enhance the uptake of nutrients. Porcine hepatocytes express the same metabolic, secretory, and immune regulatory functions as human hepatocytes. Porcine hepatocytes metabolize drugs and toxins via the cytochrome P450 enzyme system, synthesize albumin, coagulation factors, and bile acids, and secrete bile via bile canaliculi into the intestine for fat digestion. In certain cases, porcine hepatocytes can also express α-1-antitrypsin to help regulate the immune system.
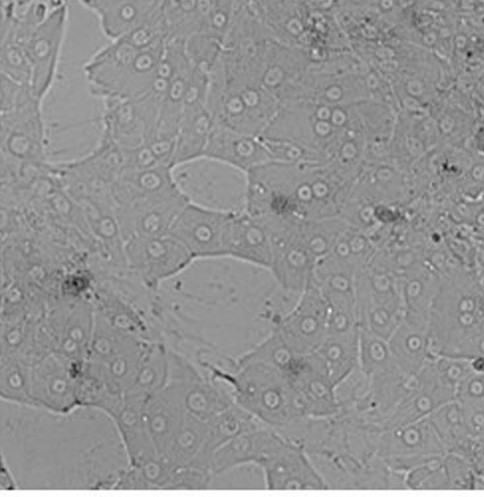 Fig. 1. Primary hepatocytes from pig seeded on a collagen layer directly after cell isolation (Andres S, Bartling B, et al., 2023).
Fig. 1. Primary hepatocytes from pig seeded on a collagen layer directly after cell isolation (Andres S, Bartling B, et al., 2023).
VPA Induced Primary Porcine Hepatocytes to Reprogram into Hepatic Progenitor Cells In Vitro
Hepatocyte transplantation is considered a feasible and cost-effective alternative to liver transplantation for end-stage liver disease. Human hepatocytes are limited and porcine hepatocytes are a potential alternative. However, the long-term in vitro proliferation of porcine hepatocytes is restricted.
Li's team developed a method to stimulate the in vitro proliferation of primary porcine hepatocytes using Valproic acid (VPA) (Fig. 1a). The growth-stimulating medium was named VPA-HCM, and it contained 0.5 mM VPA added to HCM. Primary pig hepatocytes (PPH) were rapidly proliferated in VPA-HCM (Fig. 1c) and the nucleus-to-cytoplasm ratio was increased at seven days compared to that of primary pig liver cells (Fig. 1b). Immunofluorescence staining showed that compared with freshly isolated pig liver cells, cells cultured in VPA-HCM for seven days expressed significantly higher cell cycle marker (MKI67 and PCNA) and HPC marker (KRT19 and SOX9) and hepatocyte function marker (HNF1A, HNF4A and Albumin) was decreased (Fig. 1d, e). The cell morphology changed from that of a typical hepatocyte to that of a progenitor cell after 7 days of culture in VPA-HCM, and they were called Valproic acid-induced Hepatic Progenitor Cells (VPA-iHPCs). VPA-iHPCs (D7) upregulated HPC and cell cycle genes and downregulated mature hepatocyte function genes in the qPCR assay (Fig. 1f). In summary, VPA-induced the differentiation of primary porcine hepatocytes into high-proliferation HPCs.
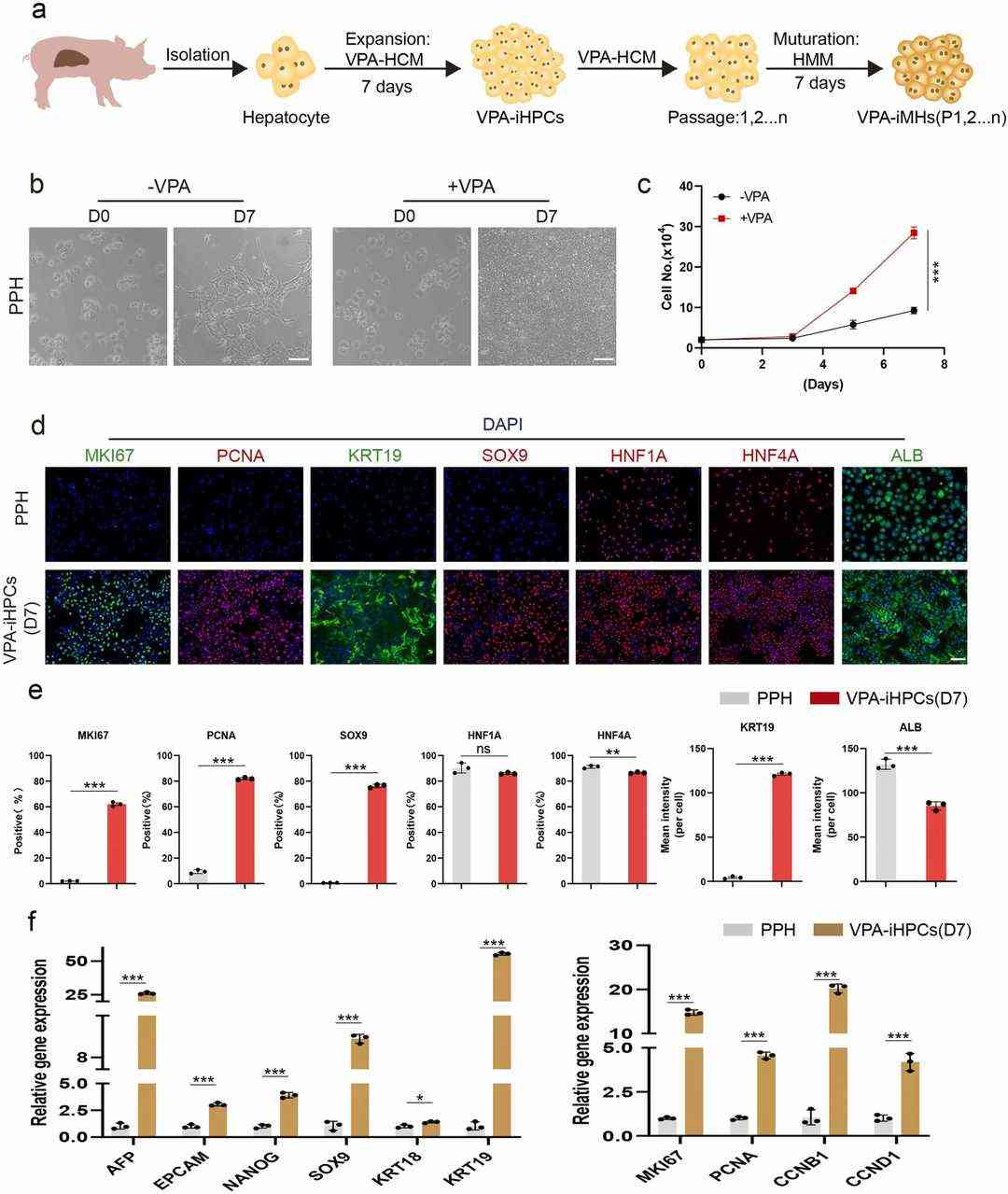 Fig. 1. VPA induced primary porcine hepatocytes to reprogram into Hepatic Progenitor Cells in vitro (Li G H, Zeng M, et al., 2024).
Fig. 1. VPA induced primary porcine hepatocytes to reprogram into Hepatic Progenitor Cells in vitro (Li G H, Zeng M, et al., 2024).
Traversal of Porcine Hepatocytes by Plasmodium falciparum Sporozoites
Plasmodium falciparum (Pf) causes severe malaria by infecting human hepatocytes. The molecular mechanisms of host specificity and maturation in non-human hepatocytes are poorly understood. Here, van der Boor et al. used fresh porcine hepatocytes to study Pf sporozoite invasion and development.
Different seeding densities were tested, with viability assessed using brightfield microscopy and MTT from the day of invasion to day five (Fig. 2A and B). Metabolic activity, measured by MTT, increased up to 72 hours post-invasion for all densities and then stabilized (Fig. 2B). Beyond day seven, cell viability decreased, showing clumping under microscopy. An optimal density of 62,500 was selected, as higher densities led to cell clumping rather than monolayer formation, aligning with densities used for primary human hepatocytes. Both human and porcine hepatocytes were infected with NF135.C10 sporozoites two days post-plating. After a three-hour traversal assay, significant FITC dextran-positive cells indicated NF135.C10 sporozoites could traverse porcine hepatocytes, though less efficiently than human ones. Traversal was less in porcine than in human hepatocytes (Fig. 2C and D).
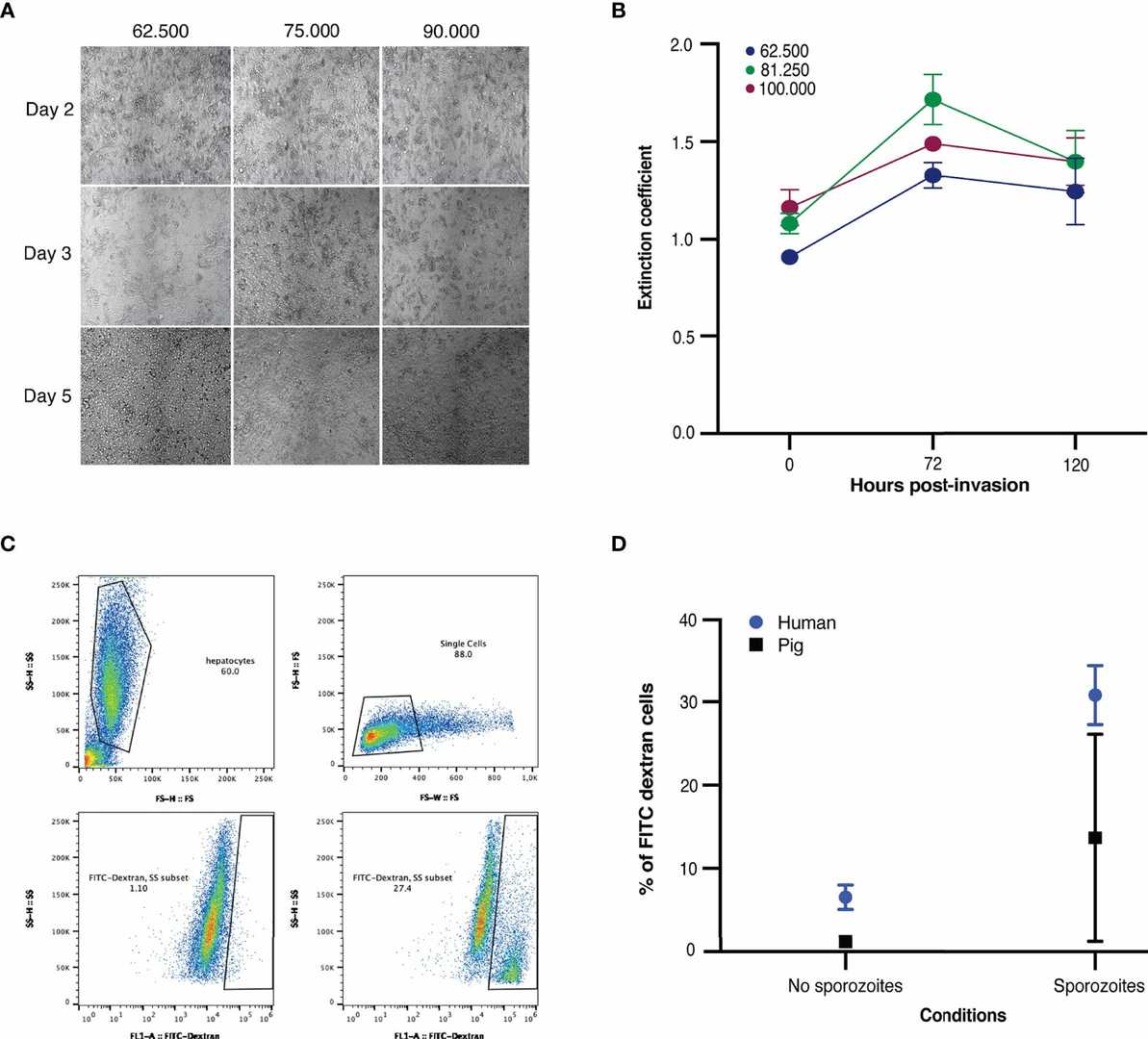 Fig. 2. (A-B) Microscopic images and viability of freshly seeded porcine hepatocytes. (C-D) Traversal of NF135.C10 sporozoites in fresh human and porcine hepatocytes three hours post-invasion of three biological donors (van der Boor SC, van Gemert GJ, et al., 2022).
Fig. 2. (A-B) Microscopic images and viability of freshly seeded porcine hepatocytes. (C-D) Traversal of NF135.C10 sporozoites in fresh human and porcine hepatocytes three hours post-invasion of three biological donors (van der Boor SC, van Gemert GJ, et al., 2022).
Most of the general culture media use HCO3-/CO32-/H+ as the pH buffer system, and the amount of NaHCO3 in the medium will determine the concentration of CO2 that should be used for cell culture. When the NaHCO3 content in the medium is 3.7 g per liter, 10% CO2 should be used for cell culture; when the NaHCO3 content in the medium is 1.5 g per liter, 5% CO2 should be used for cell culture.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Trustworthy products
We all agree that the quality of the products is good and trustworthy.
02 Aug 2023
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need