Porcine Kidney Endothelial Cells
Cat.No.: CSC-C8638W
Species: Pig
Source: Kidney
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Porcine Kidney Endothelial Cells (PKEC) are primary endothelial cells obtained from the microvascular bed of the kidney. Kidney tissue is commonly obtained from healthy, 30 kg Landrace pigs, with perfusion of the tissue through the renal artery using a Liberase enzyme solution to release endothelial cells from glomerular capillaries and peritubular vessels. PKEC attach and grow as a confluent "cobblestone" monolayer with typical endothelial morphology (10-15 µm diameter, round nuclei) and expression of key endothelial markers (CD31 (PECAM 1), von Willebrand factor, VE cadherin, ESAM) under standard cell culture conditions (37 °C, 5 % CO₂).
Functionally, PKEC are able to form tube‑like structures in Matrigel, they maintain high trans‑endothelial electrical resistance and demonstrate regulated permeability to macromolecules, indicative of a functional vascular barrier. They also show appropriate regulation of tight‑junction proteins (ZO‑1, claudin‑5) and adhesion molecules (ICAM‑1, VCAM‑1) in response to both VEGF and inflammatory stimuli. PKEC have also been used as an in vitro model for xenotransplantation, where genetic modifications (ST8Sia6 over‑expression, GTKO background) to the endothelial cells significantly decrease cytotoxicity of human peripheral blood mononuclear cells, providing a model to assess human compatibility of pig kidneys. Other applications of PKEC include organ preservation, antiviral studies (e.g. PCV2 infection), screening for anti‑angiogenic or anti‑inflammatory drugs and tissue‑engineering strategies to recellularize decellularized kidney scaffolds with endothelium.
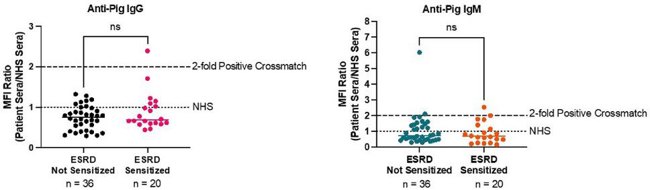
Assessing Compatibility for Xenotransplantation: Human Serum with Gene Edited Porcine Cells
Xenotransplantation is a potential solution to the organ shortage crisis. However, compatibility is a big issue because preformed antibodies against pig antigens can cause severe rejection. Getchell et al. developed a cross-match test using patient sera and gene-edited gene-edited porcine kidney endothelial cells (KEC) to see if highly sensitized patients are more likely to have antibodies that react with pig antigens.
They collected serum samples from 56 patients (both sensitized and non-sensitized) and tested them against pig kidney endothelial cells from genetically modified pigs. The test involved incubating the sera with the cells and then using secondary antibodies to detect human IgG and IgM. Results were measured using flow cytometry, and a positive cross-match was defined by a ≥2-fold increase in MFI ratio compared to normal human serum. Results showed that only 1 patient (1.8%) had anti-pig IgG antibodies, and 4 patients (7.1%) had anti-pig IgM antibodies. Sensitized and non-sensitized patients had similar cross-match results (Fig. 1). Overall, most highly sensitized patients did not have antibodies that reacted with pig antigens, suggesting that HLA sensitization is not a major barrier to xenotransplantation. This means xenotransplantation could be a good option for patients who struggle to find compatible human donors.
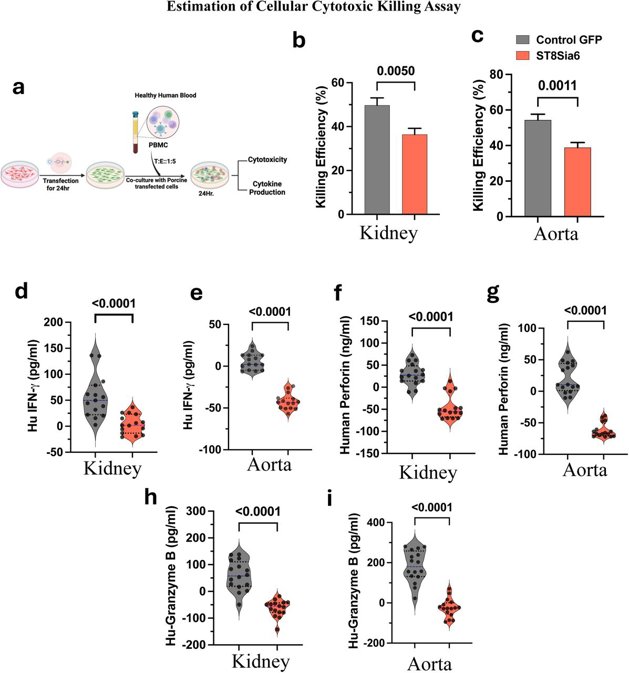
Immune Cell Cytotoxic Effects are Blunted in ST8Sia6 Expressing Porcine Kidney and Aorta Endothelial Cells
The organ shortage crisis leaves over 100,000 people waiting for transplants, causing 6,000 deaths annually. Pigs are being explored as potential donors, but immunological challenges remain. Roy et al. investigated the use of human sialyltransferase (ST8Sia6) in porcine endothelium to create human-like sialic linkages, aiming to reduce immune responses and improve xenograft survival.
Porcine kidney and aorta endothelial cells with ST8Sia6 expression were exposed to human peripheral blood mononuclear cells (PBMCs). Expression of ST8Sia6 in porcine endothelial cells protected them from human immune system rejection, and they therefore hypothesized that ST8Sia6 expression could be protective against immune mediated xenograft rejection (Fig. 2a). They then tested if ST8Sia6 could reduce cytotoxic effects from PBMCs. Co-culture assays showed that ST8Sia6-expressing cells had significantly reduced susceptibility to PBMC-induced cytotoxicity compared to controls (Fig. 2b, c). They also observed a significant reduction of IFN-γ, perforin and granzyme-B cytokine levels in the supernatants of the PBMC co-culture assays involving the cells transfected with ST8Sia6 when compared to control cells (Fig. 2d-i). These findings highlight the fact that expression of ST8Sia6 has the ability to significantly modulate the cytotoxic activities of the human immune cells.
After taking out the frozen tube, put it into the 37°C water tank immediately to thaw it quickly and shake it gently to make it thaw within 1 minute. In addition, when taking out the frozen tube from the liquid nitrogen barrel to thaw, attention must be paid to safety to prevent the bursting of the frozen tube.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Pleasant in after-taste
Good experimental results and high utilization rate after use.
12 Mar 2023
Ease of use
After sales services
Value for money
Write your own review