Human Pulmonary Microvascular Endothelial Cells
Cat.No.: CSC-C1551
Species: Human
Source: Lung
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human pulmonary microvascular endothelial cells (HMVECs) originate from human lung tissue located in peripheral areas such as the alveolar septa and pulmonary microvascular regions. The high vascular endothelial characteristics in HPMEC result from the dense network of blood flow and lymphatic vessels within lung tissue. HPMEC expresses endothelial cell markers CD31 CD144 and von Willebrand factor (vWF). Under suitable in vitro culture conditions HPMEC forms tubular structures that mimic the natural microvascular arrangement observed in living organisms. They form tight connections with pulmonary arteries veins and capillaries that help regulate lung blood flow and oxygen exchange. Additionally, these cells preserve the stability of pulmonary blood vessels and simultaneously manage vascular permeability and inflammatory control. They react to inflammatory agents such as TNF-α and LPS while modulating cell permeability and apoptosis by activating signaling pathways like JNK and RhoA/ROCK.
HPMEC is widely used in the study of lung diseases, such as acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease (COPD), and pulmonary arterial hypertension. Due to their sensitivity to inflammatory factors and oxidative stress, HPMEC are also used to assess the impact of drugs on pulmonary microvascular function.
 Fig. 1. Human lung microvascular endothelial cells (HLMVEC) in culture (Catravas JD, Snead C, et al., 2010).
Fig. 1. Human lung microvascular endothelial cells (HLMVEC) in culture (Catravas JD, Snead C, et al., 2010).
Human Pulmonary Microvascular Endothelial Cells Respond to Damps from Injured Renal Tubular Cells
Acute kidney injury (AKI) causes remote organ dysfunction, particularly affecting lungs, with mortality rates between 60%-80%. The mechanism involves damage-associated molecular patterns (DAMPs) released from injured kidney cells that bind to pattern recognition receptors (PRRs) on the pulmonary endothelium, triggering inflammatory responses. DeWolf et al. investigated the impact of DAMPs from injured renal tubular epithelial cells (RTECs) on human pulmonary microvascular endothelial cells (HMVECs) and explore potential therapeutic targets to mitigate lung injury post-AKI.
They investigated if NLR or TLR signaling genes are differently expressed in pulmonary HMVECs stimulated with supernatant from injured RTECs. Bulk RNA sequencing revealed 2,785 differentially expressed genes between HMVECs treated with necrotic supernatant and media alone (Fig. 1a). Each dot in the figure represents a gene, with blue for downregulated and red for upregulated genes, represented by log fold change (LogFC) on the x-axis. Advaita iPathway? analysis identified enriched pathways from necrotic supernatant stimulation, showing 46 NLR pathway genes (Fig. 1b) and 23 TLR pathway genes (Fig. 1c) were differentially expressed. This suggests NLRs and TLRs are involved in HMVEC response to RTEC-derived DAMPs. After discovering that necrotic supernatant upregulates NLR and TLR genes in HMVECs, they examined its impact on PRR expression. HMVECs exposed to necrotic supernatant showed increased TLR2 mRNA, unchanged TLR4, unchanged NOD1 mRNA, and a significant rise in NOD2 mRNA (Fig. 2a). FACS analysis revealed increased TLR2 protein, consistent with mRNA results, while TLR4 protein remained low, regardless of stimulation (Fig. 2b). Although baseline NOD1 expression was high, it did not increase with stimulation. NOD2 protein levels were minimal in both groups, despite a notable NOD2 mRNA increase, indicating complex transcriptional control for further study. NLRP3 was highly expressed at baseline but did not significantly change with necrotic supernatant stimulation.
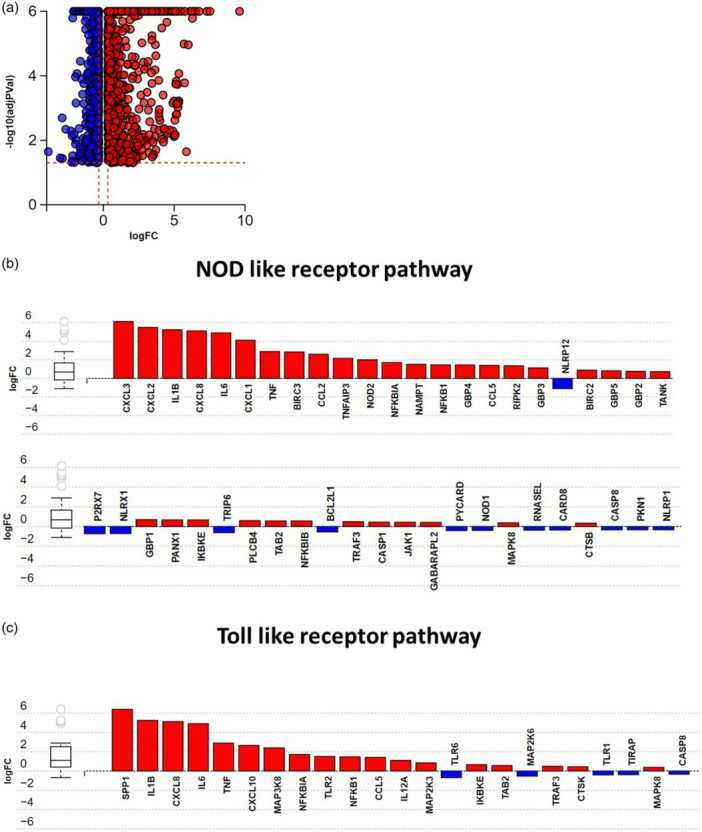 Fig. 1. Necrotic supernatant from renal tubular epithelial cells causes activation of NLR and TLR signaling pathways in HMVECs (DeWolf SE, Hawkes AA, et al., 2024).
Fig. 1. Necrotic supernatant from renal tubular epithelial cells causes activation of NLR and TLR signaling pathways in HMVECs (DeWolf SE, Hawkes AA, et al., 2024).
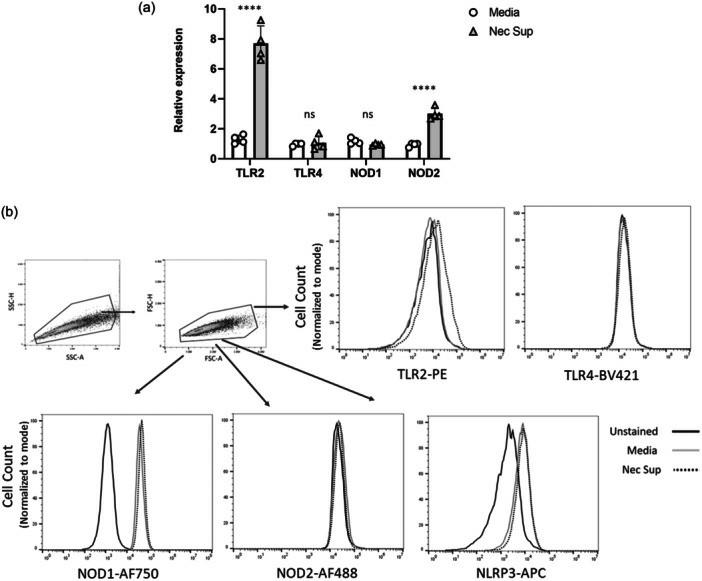 Fig. 2. Supernatant from injured RTECs causes upregulation of NOD2 and TLR2 in HMVECs (DeWolf SE, Hawkes AA, et al., 2024).
Fig. 2. Supernatant from injured RTECs causes upregulation of NOD2 and TLR2 in HMVECs (DeWolf SE, Hawkes AA, et al., 2024).
Iloprost Attenuates Oxidative Stress-Dependent Activation of Collagen Synthesis Induced by Sera from Scleroderma Patients in HMVECs
Reactive oxygen species (ROS) production by skin, visceral fibroblasts, and endothelial cells (ECs) is implicated in systemic sclerosis (SSc). SSc-related oxidative stress activates and damages endothelial cells, leading to vascular issues. As initiators of SSc vascular remodeling, ECs stimulate growth factors, cytokines, and inflammatory agents crucial for fibrosis. Excessive ECM component deposition, like collagen, results from altered synthesis or degradation, with ECs contributing to collagen synthesis. Giordo et al. showed that sera from SSc patients cause increased ROS production and collagen synthesis in human pulmonary microvascular endothelial cells (HMVECs), supporting the idea that pro-oxidant factors trigger early SSc fibrotic events in an ROS-dependent manner. ROS levels and COL1A1 activity were tracked over 4 hours (Fig. 3A–D), with first-hour data used for comparison (Fig. 3B–E). Figure 3B shows that SSc sera significantly raise ROS levels compared to healthy donor (HD) sera. Similarly, SSc sera sharply increase collagen synthesis in the first hour, more than HD sera (Fig. 3E). Iloprost maintains vascular function by enhancing endothelial junctions and reducing the shift to collagen-producing cells. Consistent with this, our data show Iloprost reduces EC damage (Fig. 3C–F) and collagen synthesis (Fig. 3E) caused by SSc sera.
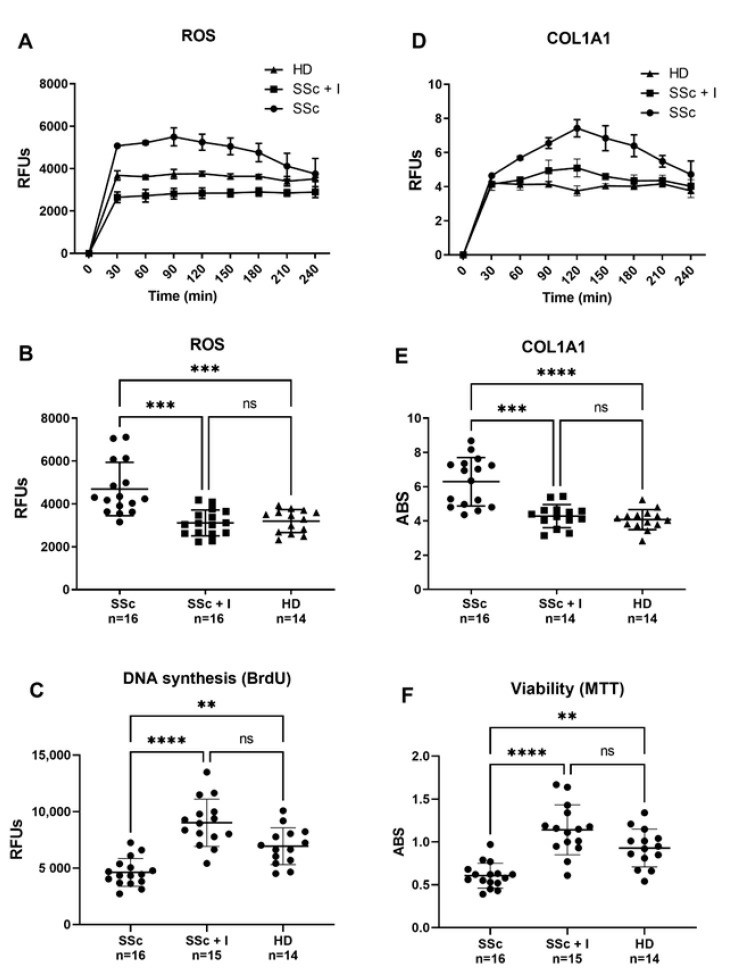 Fig. 3. Effect of SSc and SSc iloprost-treated sera on human pulmonary microvascular endothelial cells (HPMECs) intracellular ROS and COL1AI levels (A, B, D, E), DNA syntheses and viability (C, F) (Giordo R, Thuan DTB, et al., 2021).
Fig. 3. Effect of SSc and SSc iloprost-treated sera on human pulmonary microvascular endothelial cells (HPMECs) intracellular ROS and COL1AI levels (A, B, D, E), DNA syntheses and viability (C, F) (Giordo R, Thuan DTB, et al., 2021).
Ask a Question
Write your own review