
Immortalized Mouse Microglia (SIM-A9)
Cat.No.: CSC-I9208L
Species: Mus musculus
Source: Postnatal mouse cerebral cortices
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
2) ELISA was used to determine the TNFα secretion after inflammatory stimulation;
3) Western blot and immunocytochemistry were used to confirm the expression of microglial specific markers such as CD68 and Iba1 and M1/M2 phenotype-associated protein expression (COX-2/iNOS and Arg-1) after stimulation;
4) Aβ1-42orE. coli-derived bioparticles uptake was used to determine phagocytic activity of SIM-A9 cells.
Neonatal C57BL/6 mouse cerebral cortex primary glial cell cultures serve as the biological source for the derivation of the SIM-A9 cell line. These cells have naturally become immortal which enables their unending division without the need for genetic or drug-based interventions. SIM-A9 cells present multiple microglia-like shapes including spindle-shaped, multipolar, spherical and flat forms which resemble the morphologies of primary cultured microglia. The cells exhibit significant phagocytic capabilities which enable them to consume lipopolysaccharide (LPS) alongside various particulate substances. These cells release significant levels of pro-inflammatory factors including TNF-α, IL-6, and IL-1β when exposed to inflammatory agents like LPS and Aβ1-42. SIM-A9 cells possess P2X4R and BDNF receptors which respond to ATP stimulation and trigger the BDNF signaling pathway that plays a critical role in chronic neuropathic pain processes.
SIM-A9 cells demonstrate polarization into M1 (pro-inflammatory) and M2 (anti-inflammatory) states which makes them perfect for studying microglial polarization mechanisms. These cells allow researchers to examine neuroinflammation-related signaling pathways and molecular mechanisms including LPS-induced oxidative stress and cytokine release while assessing potential anti-inflammatory drug effectiveness. Additionally, these cells replicate neurodegenerative disease processes including Alzheimer's disease while they investigate gene expression and signaling pathways through qRT-PCR and co-immunoprecipitation methods and they are combined with neurons to study microglia-neuron interactions.
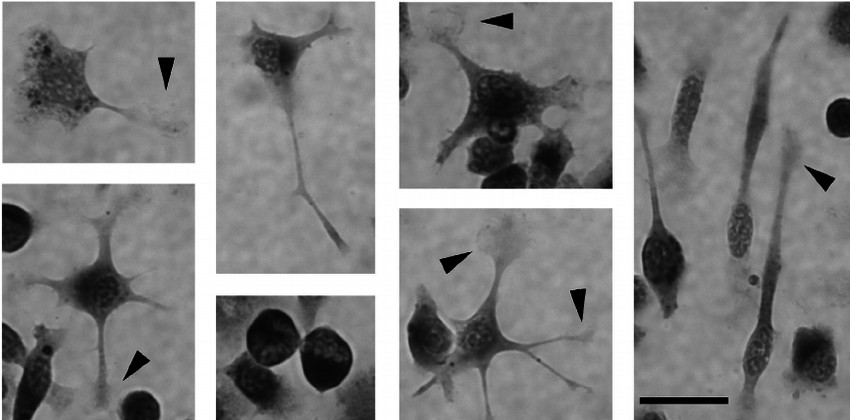 Fig. 1. High magnification photomicrographs of SIM-A9 cells representing multiple microglia-like morphologies (Nagamoto-Combs K, Kulas J, et al., 2014).
Fig. 1. High magnification photomicrographs of SIM-A9 cells representing multiple microglia-like morphologies (Nagamoto-Combs K, Kulas J, et al., 2014).
Alterations in RTP4 Expression Following LPS Treatment in SIM-A9 Microglial Cell Line
Receptor transporter protein 4 (RTP4), known for its role as a receptor chaperone for class A GPCRs, has emerged as an important player in inflammatory regulation, especially in peripheral immune responses. As microglia are key players in neuroinflammation, understanding RTP4's role is vital. By examining RTP4 and inflammation-related gene expression changes in response to LPS, Fujita et al. aims to uncover RTP4's function as an inflammation-responsive molecule in microglia.
Quantitative PCR reveals that 24 hours of LPS treatment significantly elevates RTP4 mRNA levels in a concentration-dependent manner (Fig. 1A). RTP4 upregulation is robust from 3 to 24 hours post-treatment (Fig. 1B). LPS does not significantly affect other RTPs but slightly increases RTP3, specifically upregulating RTP4 (Fig. 1C). Immunofluorescence analysis shows enhanced RTP4 expression in SIM-A9 microglial cells treated with LPS for 6, 12, and 24 hours, but not at 48 hours (Fig. 2, upper panel). Data quantification confirms increased RTP4 immunoreactivity at these times (Fig. 2, lower panel). Thus, LPS temporarily elevates RTP4 mRNA and protein levels (Fig. 2).
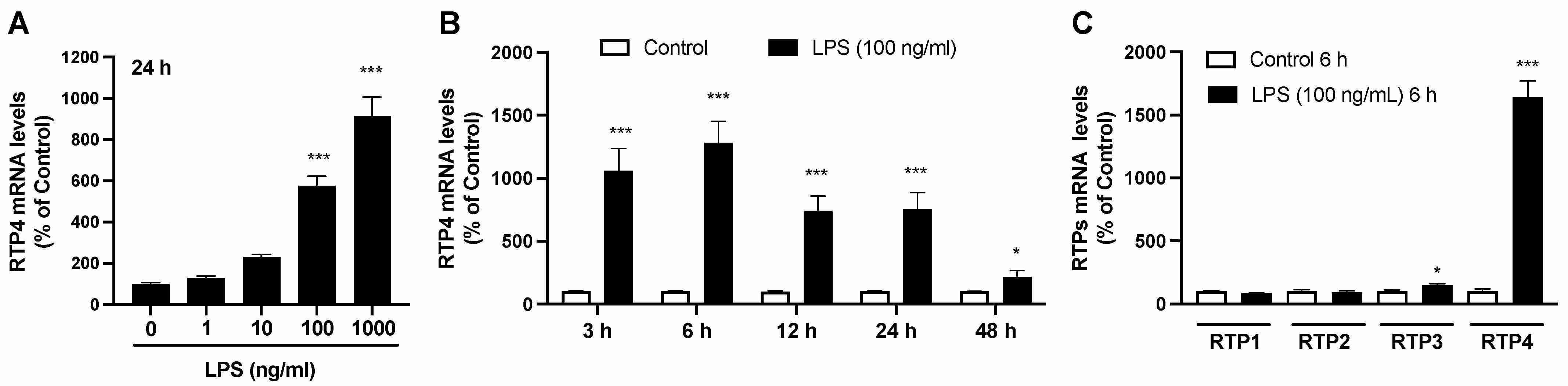 Fig. 1. Changes in RTP4 mRNA levels after LPS stimulation in SIM-A9 microglial cell line (Fujita W and Kuroiwa Y, 2024).
Fig. 1. Changes in RTP4 mRNA levels after LPS stimulation in SIM-A9 microglial cell line (Fujita W and Kuroiwa Y, 2024).
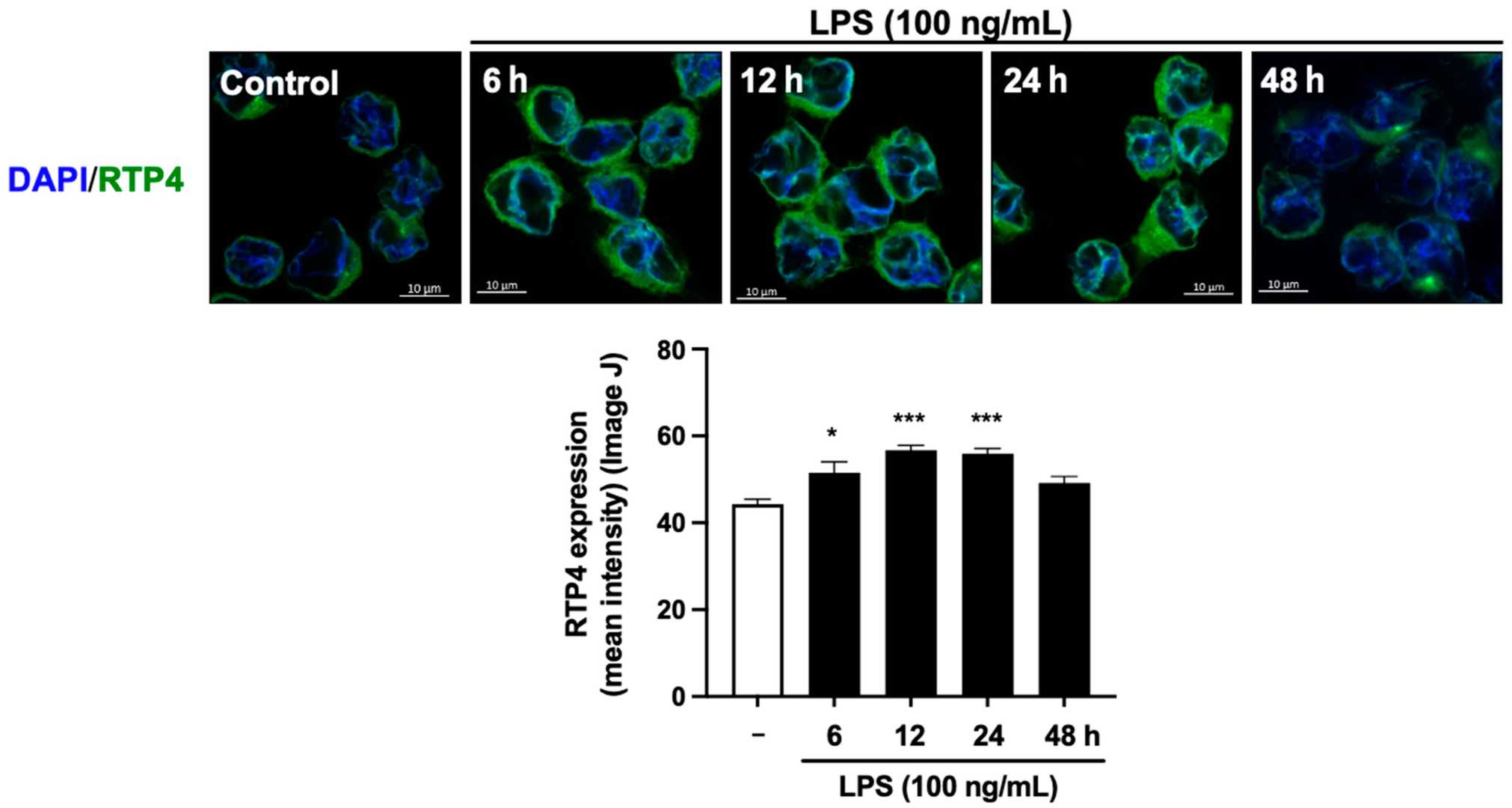 Fig. 2. The effect of LPS stimulation on the expression levels of RTP4 determined by immunofluorescent analysis (Fujita W and Kuroiwa Y, 2024).
Fig. 2. The effect of LPS stimulation on the expression levels of RTP4 determined by immunofluorescent analysis (Fujita W and Kuroiwa Y, 2024).
Impacts of HJE and luteolin on pro-inflammatory cytokines and pro-inflammatory intermediaries in SIM-A9 microglia
Humulus japonicus (HJ) possesses anti-inflammatory and antioxidant properties which make it a traditional remedy for skin and respiratory conditions while also demonstrating possible neuroprotective benefits. Wang et al. investigates how HJ ethanol extract (HJE) and its component luteolin reduce inflammation in microglial cells (SIM-A9) activated by LPS.
Microglia defend the CNS through their activation of immune responses. Abnormal activation of these cells leads to the production of pro-inflammatory intermediaries (ROS, COX2, PGE2, NO, iNOS) and cytokines (IL-1β, TNF-α, IL-6) which results in neurodegenerative diseases like Parkinson's and cognitive decline. Controlling neuroinflammation is vital for treatment. Microglial activation through LPS stimulation results in the production of neurotoxic intermediaries which lead to neuron death. Our research demonstrates that LPS by itself elevates NO, iNOS, PGE2, COX2 levels (Fig. 3), and cytokine production (IL-1β, TNF-α, IL-6) (Fig. 4). When cells receive pretreatment with HJE and luteolin before receiving LPS exposure, they show limited increases in levels of inflammatory cytokines and intermediaries. SIM-A9 microglial cells treated with LPS show reduced inflammation when pretreated with HJE and luteolin.
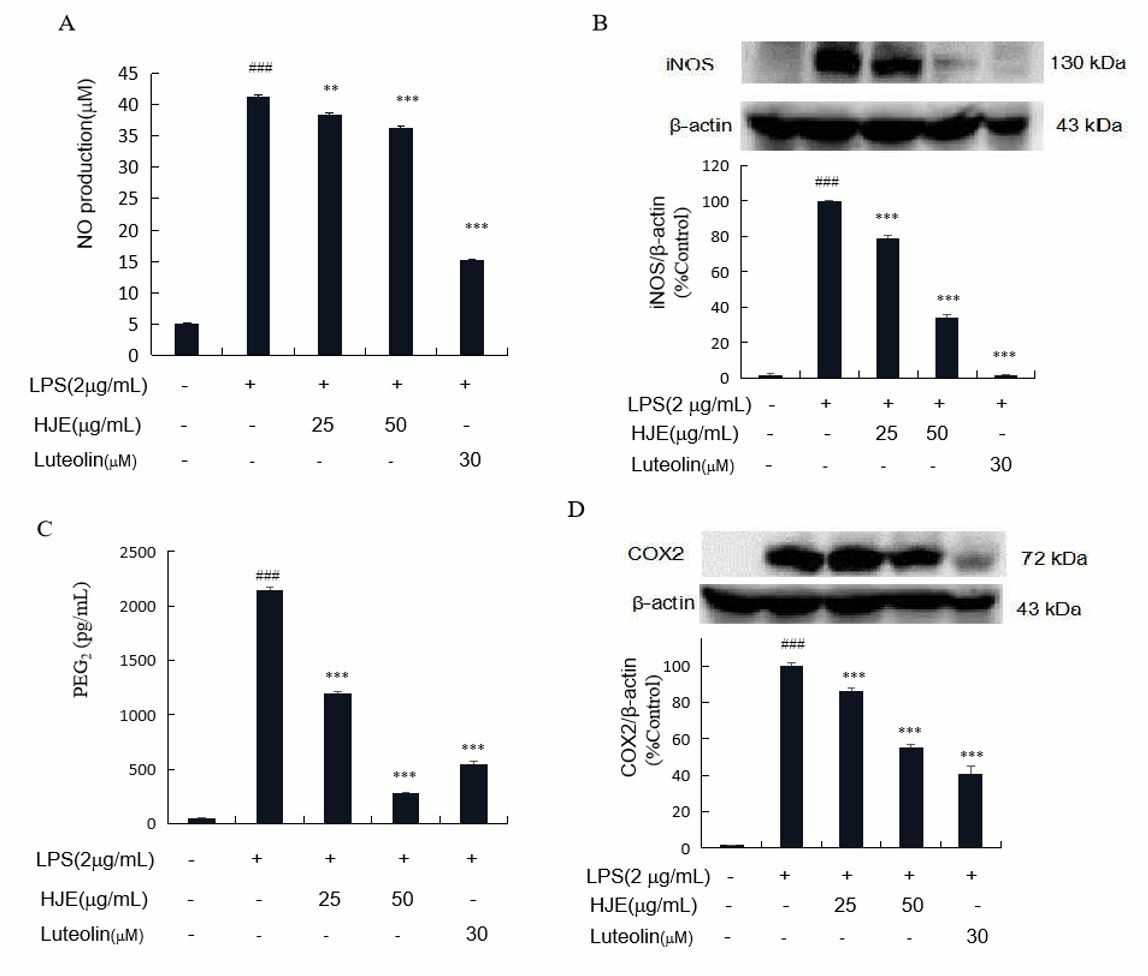 Fig. 3. The effects of HJE and luteolin NO production (A), iNOS expression levels (B), PGE2 generation (C), and COX2 expression degrees (D) in LPS-stimulated microglia cells SIM-A9 (Wang F, Cho B O, et al., 2022).
Fig. 3. The effects of HJE and luteolin NO production (A), iNOS expression levels (B), PGE2 generation (C), and COX2 expression degrees (D) in LPS-stimulated microglia cells SIM-A9 (Wang F, Cho B O, et al., 2022).
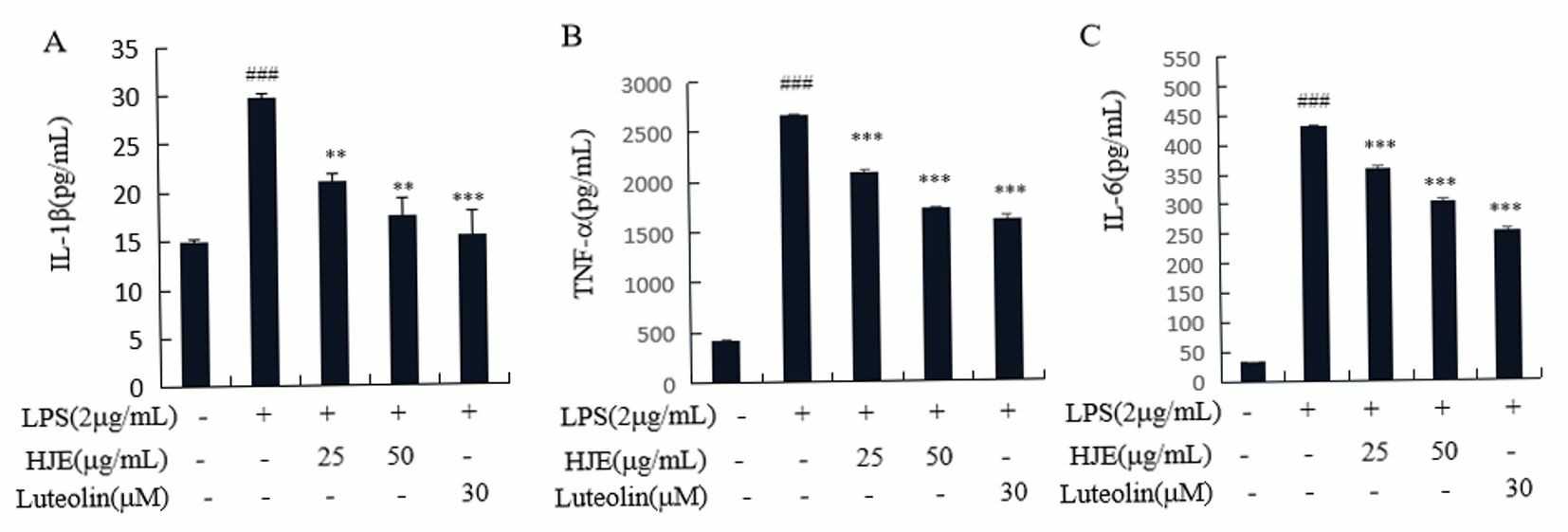 Fig. 4. Impacts of HJE and luteolin on IL-1β(A), TNF-α(B) along with IL-6 (C) production in the microglial cells stimulated by LPS (Wang F, Cho B O, et al., 2022).
Fig. 4. Impacts of HJE and luteolin on IL-1β(A), TNF-α(B) along with IL-6 (C) production in the microglial cells stimulated by LPS (Wang F, Cho B O, et al., 2022).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

