
Immortalized Mouse Cerebellar Capillary Endothelial Cells (cerebEND)
Cat.No.: CSC-I9185L
Species: Mus musculus
Source: Cerebellar cortex
Morphology: spindle shaped
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
2) Tight junction protein Claudin-5 assessed by immunostainning and claudin-1, -3 and -12 detected in mRNA levels;
3) BBB marker protein Glut-1 and tight junction-associated protein occludin measured by western blot analysis.
The cell line's response to pro-inflammatory stimuli was measured by 1) TER, 2) western blot and 3) gene expression analysis using RT-PCR.
Immortalized Mouse Cerebellar Capillary Endothelial Cells (cerebEND) is a classic in vitro blood‑brain barrier (BBB) model. The endothelial cell line was isolated from primary capillary endothelial cells from the cerebellum of neonatal (0-2 days old) mice and immortalized with the polyoma middle‑T antigen, which provides for unlimited proliferation while maintaining important endothelial phenotypes. CerebEND cells are typical of an endothelial cell line with a spindle‑shaped, adherent morphology. The cells form a confluent monolayer, express canonical endothelial markers (VE‑cadherin, CD31) and tight‑junction proteins (claudin‑3, claudin‑5, occludin, ZO‑1) as well as the glucose transporter GLUT‑1, features emblematic of cerebral microvasculature. In Transwell systems, cerebEND have been shown to have TEER values between 250 and 300 Ω·cm².
The cells have been used in numerous permeability and transport studies and react to inflammatory cytokines (TNF‑α, IL‑1β) as well as hypoxic/ischemic conditions, making them a useful model system for neuroinflammation, ischemia‑reperfusion injury, and drug transport across the BBB. CerebEND has also been used in co‑culture models with astrocytes, pericytes, or tumor cells to recapitulate the neurovascular unit. The line has become a classic in vitro BBB model because it is easy to culture, has well defined growth requirements (high‑glucose DMEM + 10 % FBS, gelatin surface coating), and a robust phenotype.
TTFields Effects on the BBB Were Dependent on the Frequency, Intensity, and Duration of the Treatment
The majority of therapeutic agents for CNS disorders are unable to penetrate the BBB, and thus their clinical effectiveness is extremely limited. Salvador et al. determined the effects of the clinically approved TTFields for GBM on blood-brain barrier permeability and integrity, which could also enhance CNS drug delivery.
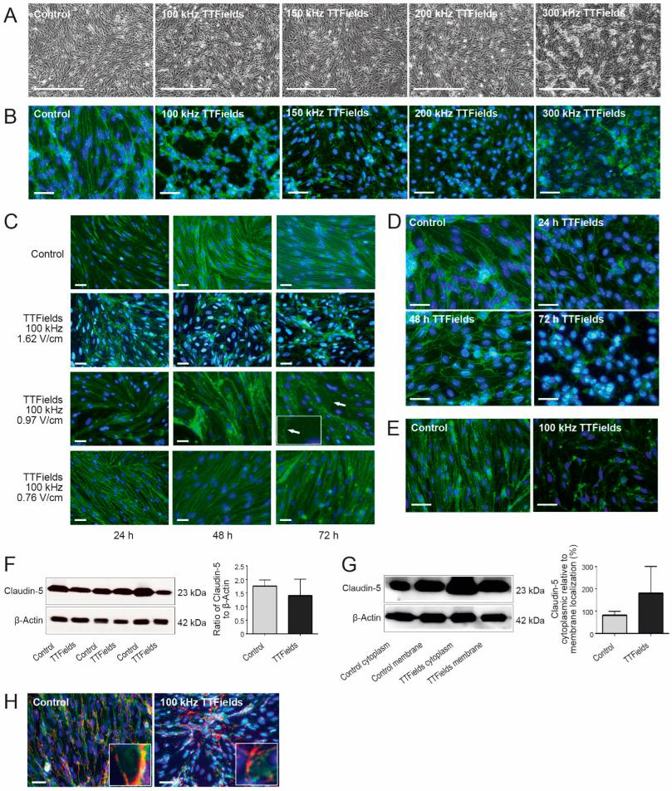
To understand how TTFields affect the blood-brain barrier (BBB), they examined mouse cerebellum-derived endothelial cells (cerebEND) under different TTFields frequencies. Normally, these cells grow in a parallel, elongated pattern. But with TTFields, they formed swirls instead (Fig. 1A). They stained tight junction proteins (claudin-5 and ZO-1) to see changes better. After TTFields treatment, cells lost their elongated shape and became irregular (Fig. 1B). All tested frequencies (100-300 kHz) changed cell shape, but 100 kHz had the most visible effect. So, they used 100 kHz for later experiments. They also tested different intensities (1.62, 0.97, and 0.76 V/cm RMS) to see if they affected BBB opening. At 0.76 V/cm RMS, cells looked normal but had frayed edges. At 0.97 V/cm RMS, cells deformed, losing their elongated shape. The most dramatic changes were at 1.62 V/cm RMS, where claudin-5 distribution was most disturbed (Fig. 1C). This shows that cell response depends on intensity. Next, they tested how long it takes for 100 kHz, 1.62 V/cm TTFields to be most effective. Cells were treated for 24, 48, and 72 hours. Disruption was seen after 24 hours, but the most significant changes were after 72 hours (Fig. 1D).
Alterations in Tight Junction Proteins Expression After Oxygen-Glucose Deprivation in Murine Brain Capillary Cerebellar Endothelial Cells (cerebEND)
Stroke is the second leading cause of death and contributes to neuronal damage by inducing BBB failure and microvascular disruption, which is associated with edema. Stevioside, and its metabolite isosteviol sodium (STVNA), has been previously shown to exert neuroprotective effects in cerebral ischemia, but its effects on the BBB are not known. Here, Rösing et al. determined the effect of STVNA on the BBB in an in vitro stroke model, using oxygen and glucose deprivation (OGD), in murine brain capillary cerebellar endothelial cells (cerebEND).
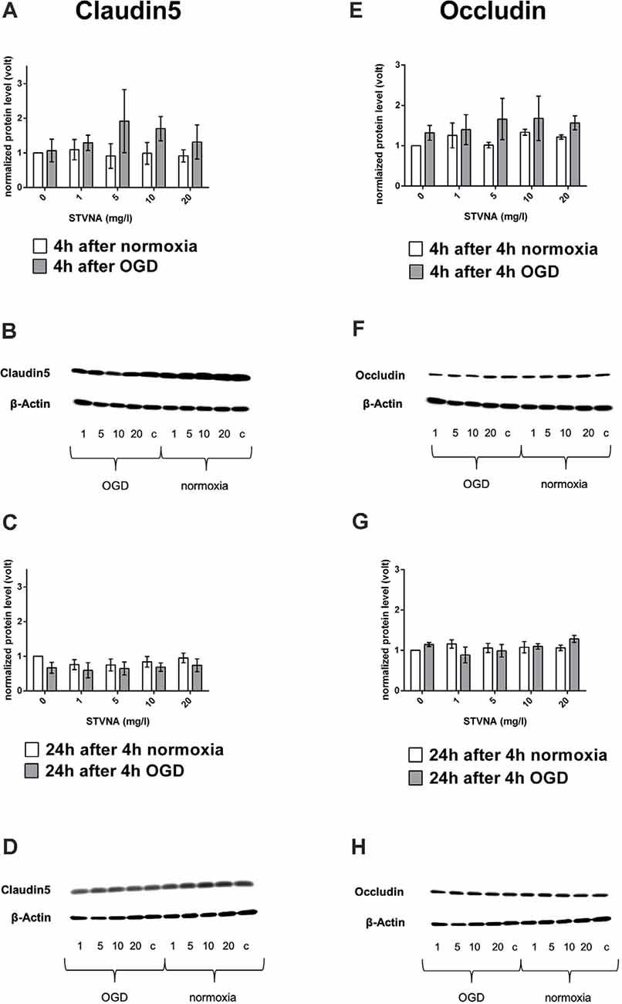
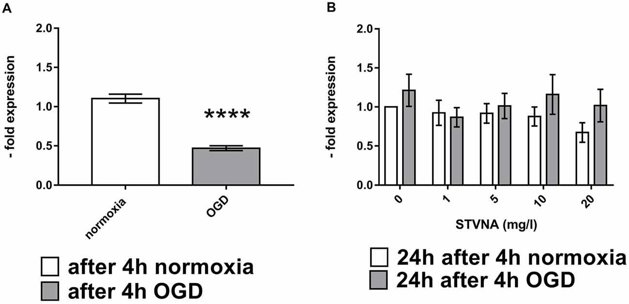
Tight junction proteins (TJPs) are crucial for the barrier properties of brain capillary endothelial cells in the BBB. When the BBB is damaged, such as during ischemia, TJP expression can change. To study this, cerebEND cells were subjected to oxygen-glucose deprivation (OGD) and then treated with various concentrations (0, 1, 5, 10, and 20 mg/l) of isosteviol sodium (STVNA) for 4 and 24 hours. Western blot analysis showed that claudin-5 expression increased after 4 hours of STVNA treatment, with 5 mg/l yielding the highest increase (Fig. 2A, B). However, after 24 hours of STVNA treatment, claudin-5 expression decreased (Fig. 2C, D). Similar trends were observed for occludin (Fig. 2G, H), with the highest increase after 4 hours of STVNA treatment at both 5 and 10 mg/l (Fig. 2E, F). Additionally, qRT-PCR analysis showed that 24 hours of STVNA treatment post-OGD increased claudin-5 mRNA expression for all tested concentrations (Fig. 3).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells
