KYSE-520
Cat.No.: CSC-C0419
Species: Homo sapiens (Human)
Source: Esophagus
Morphology: epitheloid cells growing adherently as monolayers, occasional giant cells present (ca. 1%)
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: cytokeratin +, cytokeratin-7 -, cytokeratin-8 +, cytokeratin-17 +, cytokeratin-18 +, cytokeratin-19 +, desmin -, endothel -, EpCAM +, GFAP -, neurofilament -
KYSE-520 is a well-established human esophageal squamous cell carcinoma (ESCC) cell line, originally derived from a primary tumor of a 66-year-old male patient in the 1990s. It retains key molecular and phenotypic features of ESCC, including epithelial morphology, proliferation as an adherent monolayer, and characteristic genetic alterations such as TP53 mutations and CDKN2A deletions, which are common drivers of esophageal cancer pathogenesis. These cells also exhibit dysregulated signaling pathways (e.g., EGFR, PI3K/AKT) and express markers like cytokeratins, making them valuable for studying mechanisms of tumorigenesis, metastasis, and drug response.
KYSE-520 cells are moderately differentiated and useful for studying epithelial-mesenchymal plasticity (EMP) in esophageal cancer. They exhibit heterogeneity, consisting of both epithelial-like (CD44v+) and mesenchymal-like (CD44v-) subpopulations. These two populations are able to interconvert, reflecting a dynamic EMP process. This property makes KYSE520 a good model for studying ESCC cancer stem cell properties and mechanisms of chemoresistance.
KYSE520 cells have been used to explore the role of fibroblast growth factor receptor-like 1 (FGFRL1) in therapeutic studies. Studies have shown that FGFRL1-deficient KYSE520 cells exhibit reduced tumor growth and motility, as well as a decrease in matrix metalloproteinase-1 (MMP-1) and fibroblast growth factor binding protein 1 (FGFBP1) expression. These findings highlight the importance of FGFRL1 in tumorigenesis and suggest potential therapeutic targets. In addition, the EMP dynamics and related molecular pathways in KYSE520 cells provide insights into the mechanisms of ESCC progression and resistance, contributing to the development of targeted therapies.
TK-642 Inhibits Cell Proliferation and Induces Apoptosis of Esophageal Cancer Cells In Vitro
Src homology-2-containing protein tyrosine phosphatase 2 (SHP2) is a promising therapeutic target for cancer therapy. Pyrazolopyrazine-based TK-642 is identified as a highly potent, selective, orally bioavailable allosteric SHP2 inhibitor with favorable pharmacokinetic profiles.
The cellular thermal shift assay (CETSA) was performed to investigate the potential of TK-642 in targeting intracellular SHP2 protein. As illustrated in Fig. 1A, a significantly higher expression level of SHP2 protein was observed within the temperature range of 45-63°C in EGFR amplified KYSE-520 esophageal cancer cell lines treated with TK-642, as compared to the DMSO treatment group. This finding indicated that TK-642 could effectively bind to and enhance the thermal stability of intracellular SHP2 protein in KYSE-520 cells.
Considering the well-known oncogenic roles of SHP2 in the development of esophageal cancer, we proceeded to investigate the impact of TK-642 on cell proliferation and apoptosis of KYSE-520 cell lines, with SHP099 serving as a control. The results revealed that both compounds exhibited moderate effects on the proliferation of KYSE-520 cells. Especially, the inhibitory activity of TK-642 on the KYSE-520 cells was approximately 3-fold greater than that of SHP099 (Fig. 1B). Moreover, we also verified the anti-tumor activity of TK-642 in non-small cell lung cancer H1975 and gastric cancer HGC-27 cells (Fig. 1C). Similar to SHP099, TK-642 also induced the apoptosis of KYSE-520 cells in a concentration-dependent manner (Fig. 1D).
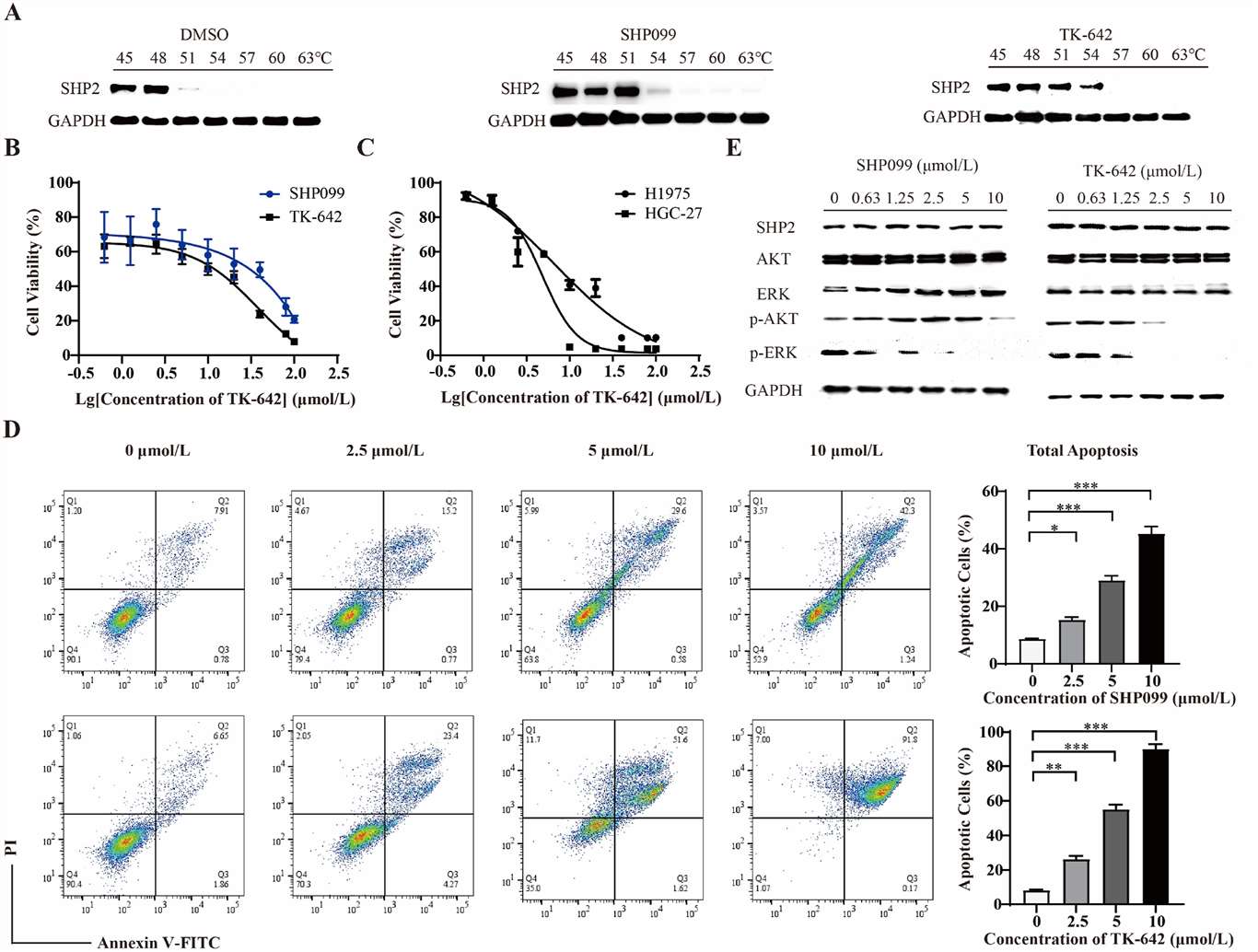 Fig. 1. TK-642 inhibits cell proliferation and induces apoptosis of esophageal cancer cells KYSE-520 (Tang, Kai, et al. 2024).
Fig. 1. TK-642 inhibits cell proliferation and induces apoptosis of esophageal cancer cells KYSE-520 (Tang, Kai, et al. 2024).
Cell Proliferation Was Reduced in Hsa_circ_0001615 Knockdown Cells
Recent studies have found that circular RNAs (circRNAs) play important roles in tumorigenesis. This study aimed to determine the function and potential mechanisms of hsa_circ_0001615 in esophageal cancer.
The endogenous expression of hsa_circ_0001615 was quantified in three esophageal cancer cell lines (ECA-109, TE-1, KYSE520) and normal esophageal cells (HEEC). The relative expression of hsa_circ_0001615 was higher in the three esophageal cancer cell lines than in normal esophageal cells. Moreover, it was higher in ECA-109 and KYSE520 cells than in TE-1 cells (Fig. 2A).
ECA-109 and KYSE520 cell lines were used to transfect hsa_circ_0001615 siRNA to gain a better understanding of the functions of hsa_circ_0001615. The relative expression of hsa_circ_0001615 was significantly reduced after transfection with siRNAs for 48 h (Fig. 2B). Cell proliferation was significantly impaired when hsa_circ_0001615 was knocked down in ECA-109 and KYSE520 cells, as demonstrated by MTS assays (Fig. 2C). Cloning assay was also performed to confirm the growth defect in hsa_circ_0001615 knockdown cell lines. The number of clone formation was substantially decreased when hsa_circ_0001615 was knocked down (Fig. 2D). These results indicated that reduced hsa_circ_0001615 expression decreased cell proliferation and colony formation in ECA-109 and KYSE520 cell lines.
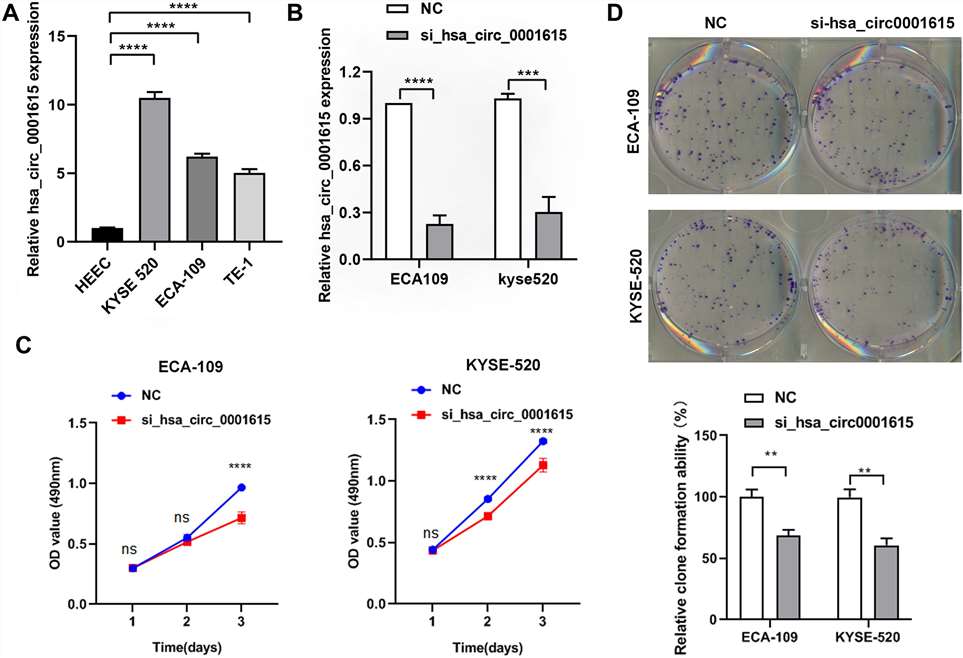 Fig. 2. Reduced cell proliferation in hsa_circ_0001615 knockdown cells (Dai, Yukai, et al. 2024).
Fig. 2. Reduced cell proliferation in hsa_circ_0001615 knockdown cells (Dai, Yukai, et al. 2024).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells





