Py8119
Cat.No.: CSC-C6529J
Species: Mus musculus (Mouse)
Source: Breast
Morphology: Epithelial-like
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Shipping: Dry Ice, Frozen
Py8119 cells, derived from a murine breast cancer model that expresses the Polyoma Middle T (PyMT) oncogene, are a widely studied cell line known for their aggressive tumorigenic and metastatic properties. These cells exhibit hallmark features of epithelial-mesenchymal transition (EMT), including decreased E-cadherin expression and increased invasiveness, enabling them to effectively model metastatic processes like lung colonization. The PyMT-driven signaling resembles the hyperactivation of pathways including PI3K/Akt and MAPK, both of which play key roles in the progression of human breast cancer.
Currently, Py8119 cells are employed to investigate mechanisms of metastasis, interactions within the tumor microenvironment, and factors contributing to therapeutic resistance. Their robust metastatic potential makes them particularly valuable for testing anti-invasive drugs such as kinase inhibitors and EMT-targeting agents, as well as for exploring combinations of immunotherapy in syngeneic mouse models. Recent studies have also leveraged Py8119 cells to identify metastasis-suppressing genes and to dissect the effects of stromal-immune cells on tumor dissemination in co-culture systems. By bridging molecular insights with preclinical validation, Py8119 cells continue to serve as an important tool for advancing targeted therapies and deepening our understanding of aggressive breast cancer sub-types.
ΔA146Ply Inhibits TNBC Cell Migration and Invasion
The detoxified pneumolysin derivative ΔA146Ply has been proven to have a direct anti-triple negative breast cancer effect, but its work model remains unclear. In this study, The researches focused on its ability to inhibit triple-negative breast cancer metastasis.
First, scratch and transwell assays were performed to verify the effect of ΔA146Ply in MDA-MB-231, py8119, and 4T1 cells. Figure 1a-c shows that the wound was wider in the ΔA146Ply group than in the medium group. Furthermore, the number of migrating and invading cells was significantly lower in the ΔA146Ply group than that in the medium group (Fig. 1d-i). These results indicate that ΔA146Ply suppressed TNBC cell migration and invasion.
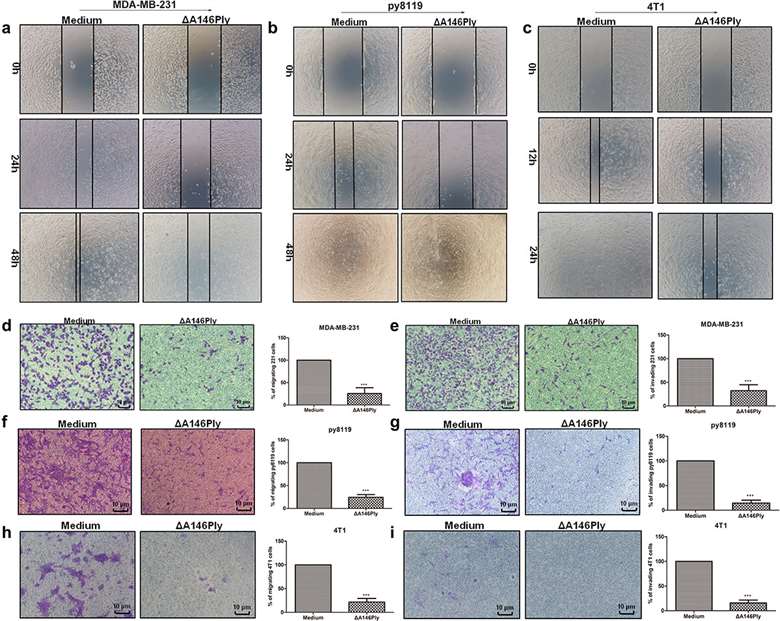 Fig. 1. ΔA146Ply inhibits TNBC cell migration and invasion (Zhang, Hong, et al. 2024).
Fig. 1. ΔA146Ply inhibits TNBC cell migration and invasion (Zhang, Hong, et al. 2024).
ARIH1 Regulates MAP4, a Microtubule-Associated Protein, in Breast Cancer Cells
Chemotherapy drugs like paclitaxel are widely used to treat breast cancer by stabilizing microtubules, structures essential for cell division. However, resistance to paclitaxel limits its effectiveness in many patients. In this study, the researchers identify ARIH1, a protein that regulates microtubule stability, as a potential therapeutic target and biomarker in breast cancer.
The researchers stably silenced ARIH1 in both SUM159 and Py8119 cells through shRNA KD and CRISPR Cas9 KO, respectively (Fig. 2A). MAP4 is a microtubule-associated protein known to regulate microtubule stability. Western blot analysis confirmed the differential expression of MAP4 in PY8119 and SUM159 cells under various conditions (Fig. 2B, C). These results suggest that ARIH1 negatively regulates MAP4 protein levels, either directly or indirectly. Immunofluorescence analysis revealed a spatial relationship between MAP4 and beta-tubulin in PY8119 and SUM159 cells (Fig. 2D). This was accompanied by the formation of prominent microtubule bundles. These findings suggest that ARIH1 deficiency leads to increased MAP4 expression and localization to microtubules.
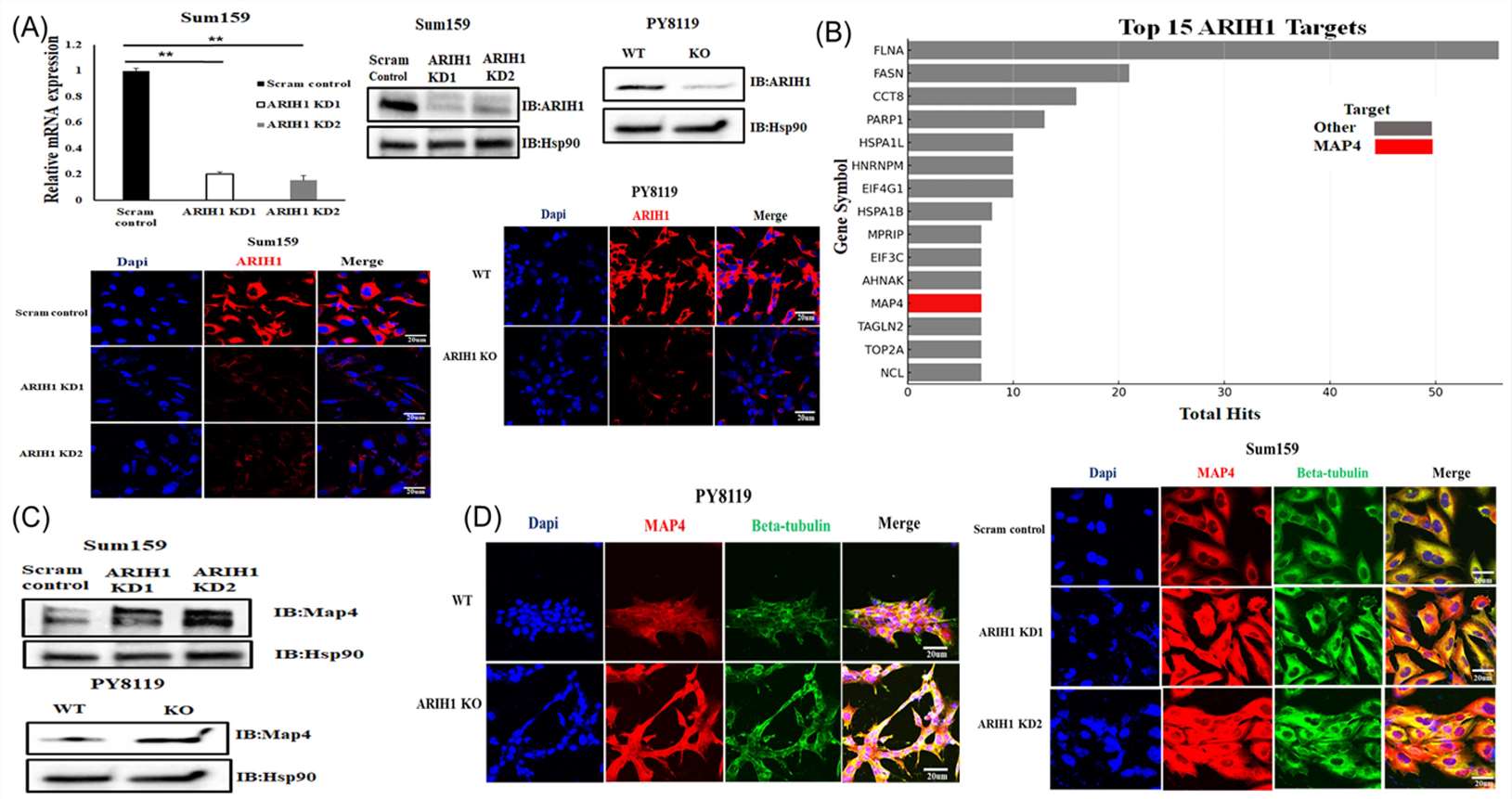 Fig. 2. ARIH1 regulates MAP4 to modulate microtubule stability (Elshaer, Mohamed, Breege V. Howley, and Philip H. Howe. 2025).
Fig. 2. ARIH1 regulates MAP4 to modulate microtubule stability (Elshaer, Mohamed, Breege V. Howley, and Philip H. Howe. 2025).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells