GFP Expressing Human Dermal Microvascular Endothelial Cells (GFP-HDMvECs)
Cat.No.: CSC-C5440W
Species: Human
Source: Dermis; Skin
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human dermal microvascular endothelial cells (HDMECs) are a key type of cell that exists in the dermis, a deep skin layer that contains an intricate network of connective tissues. This structure is packed with collagen and elastin, and it contains a thick microvascular layer in which HDMECs are essential. These cells are responsible for maintaining the integrity and function of the skin's microvascular system. HDMECs are removed from dermal tissue through certain processes, including collagenase-neutral protease digestion and density gradient centrifugation. Cultured in dedicated endothelial cell cultures, they are over 90% pure, uncontaminated with HIV-1, HBV, HCV, mycoplasma, bacteria, yeast, and fungi. They play an essential role in blood flow and vascular homeostasis and are also involved in wound healing, inflammation and angiogenesis.
GFP-expressing HDMECs from Creative Bioarray puromycin-selected following GFP-expressing lentivirus infection. These GFP-HDMECs provide distinct advantages in studying vascular physiological and pathological mechanisms, making it possible to see such intricate processes as angiogenesis, inflammation and vascularisation of tumours. Studying the behaviour and properties of these imprinted cells allows researchers to better understand the mechanisms of vascular disorders and provides a scientific basis for novel therapies and drugs.
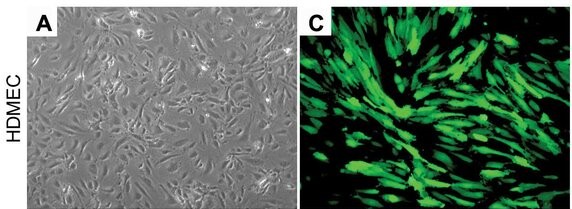 Fig. 1. Representative photomicrographs of human dermal microvascular endothelial cells (HDMEC) stably transduced with GFP under light microscopy and fluorescence microscopy (Dong Z, Imai A, et al., 2013).
Fig. 1. Representative photomicrographs of human dermal microvascular endothelial cells (HDMEC) stably transduced with GFP under light microscopy and fluorescence microscopy (Dong Z, Imai A, et al., 2013).
Dermal Fibroblasts Engulf Apoptotic Endothelial Cells In Vitro.
The effective clearance of apoptotic cells is an essential step in the resolution of healing wounds. In particular, blood vessel regression during wound resolution produces a significant number of apoptotic endothelial cells (ApoEC) that must be cleared. These cells are generally cleared out by "professional" phagocytes, including macrophages, juvenile dendritic cells and neutrophils. However, "non-professional" phagocytes, such as epithelial cells and fibroblasts, have been proposed to get involved in this process in some systems. Romana-Souza et al. explored the effect of dermal fibroblasts on skin wound healing by removing apoptotic endothelial cells (ApoEC) from the surface of wounds.
In order to find out whether dermal fibroblasts phagocytose apoptotic endothelial cells, they first looked at it in vitro. Dermal fibroblasts (HDF) treated with apoptotic GFP-HDMEC (the human dermal microvascular endothelial cells that express green fluorescent protein) over the course of 24 hours, produced vimentin-positive fibroblasts laden with apoptotic particles when fluorescence microscopy was used (Fig. 1a) and confirmed using confocal microscopy (Fig. 1b, 1c). Flow cytometry showed that 3.90.2% and 4.90.2% of HDF consumed apoptotic GFP-HDMEC, respectively, after 8 hours and 24 hours (Fig. 1d). HGF fibroblasts also phagocytosed apoptotic GFP-HDMEC (Fig. 1e). Interestingly, HDF did not swallow up viable GFP-HDMEC (Fig. 1f). These data collectively indicate that dermal fibroblasts can phagocytose apoptotic but not living endothelial cells and that the phagocytic activity of dermal fibroblasts is correlated with processes and factors that are responsible for detecting and enabling the targeted uptake of apoptotic endothelial cells.
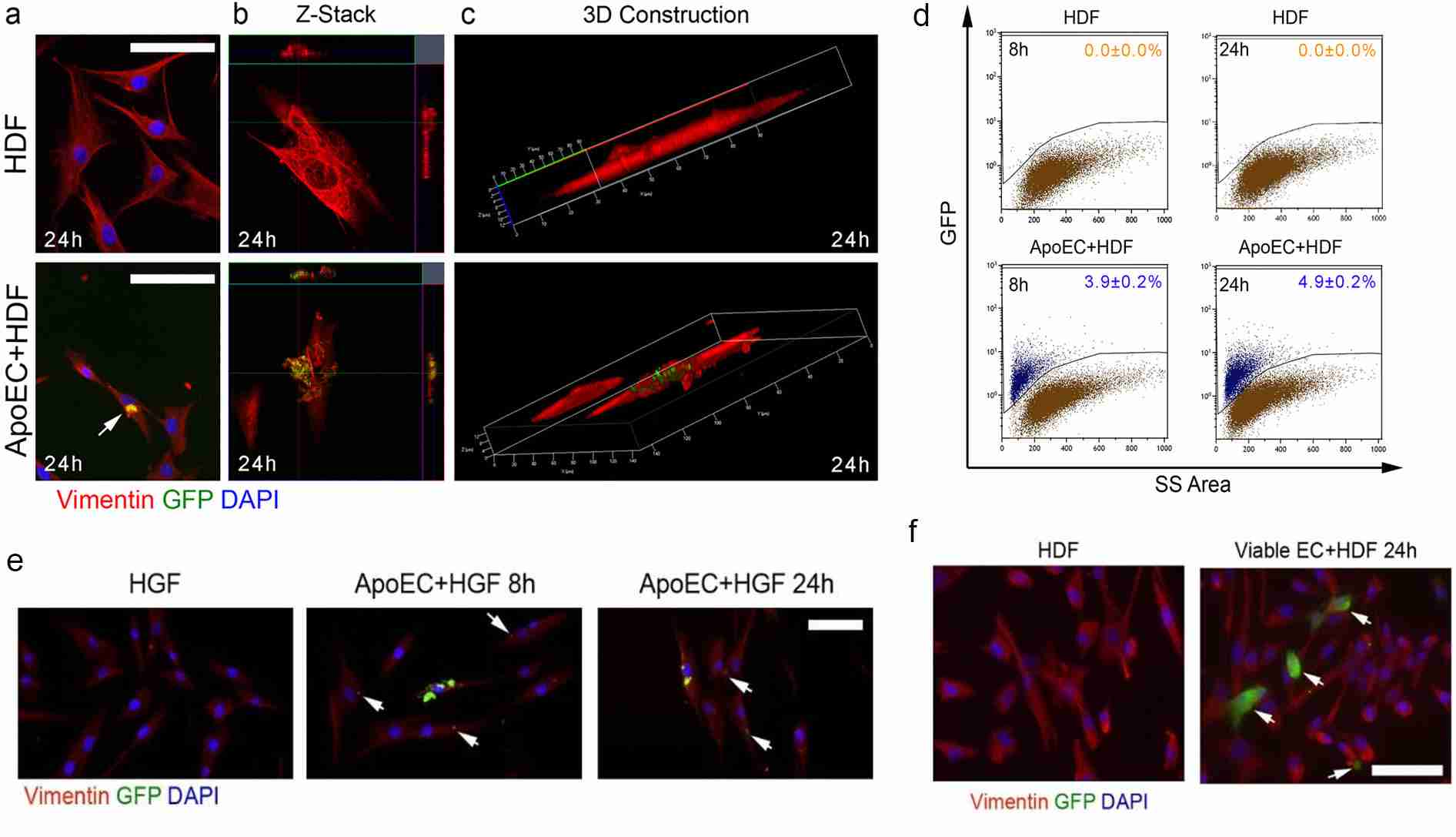 Fig. 1. Dermal fibroblasts engulf apoptotic endothelial cells in vitro (Romana-Souza B, Chen L, et al., 2022).
Fig. 1. Dermal fibroblasts engulf apoptotic endothelial cells in vitro (Romana-Souza B, Chen L, et al., 2022).
Skp2 Promoted Angiogenesis
Psoriasis is a skin disease characterised by abnormal cell growth, immune cell proliferation, and blood vessel production. The ubiquitin ligase E3 platform has been used to probe the pathogenesis of psoriasis through abnormal protein ubiquitination. Skp2, FBXL1), a member of the SCFSkp2 E3 ligase family, has been found to regulate cell growth and survival and has been implicated in many types of cancers. Xie's team examined the potential role of Skp2 in psoriasis.
To test whether Skp2 played a part in angiogenesis, they over-expressed Skp2 in HDMECs by injecting recombinant adenoviruses with Skp2 (Ad-Skp2) and a control vector (Ad-GFP) into the cells, then measured proliferation and migration using BrdU, wound healing and Boyden chamber/transwell migration. Skp2 expression by transfecting HDMECs with a recombinant adenovirus greatly enhanced BrdU incorporation, wound healing and transwell migration, indicating cell proliferation and motility (Fig. 2A-F). In addition, Skp2 overexpression also improved HDMECs' ability to form capillary-like structures on Matrigel (Fig. 2G-H). However, siRNA-induced inhibition of Skp2 also greatly slowed proliferation (Fig. 2I-J), migration (Fig. 2K-N) and tube formation (Fig. 2O-P) of HDMECs in the presence of an angiogenic stimulant, VEGF. Western blots showed that Skp2 overexpression and knockdown also worked well. Such findings suggest that Skp2 causes angiogenesis through increased endothelial cell activity.
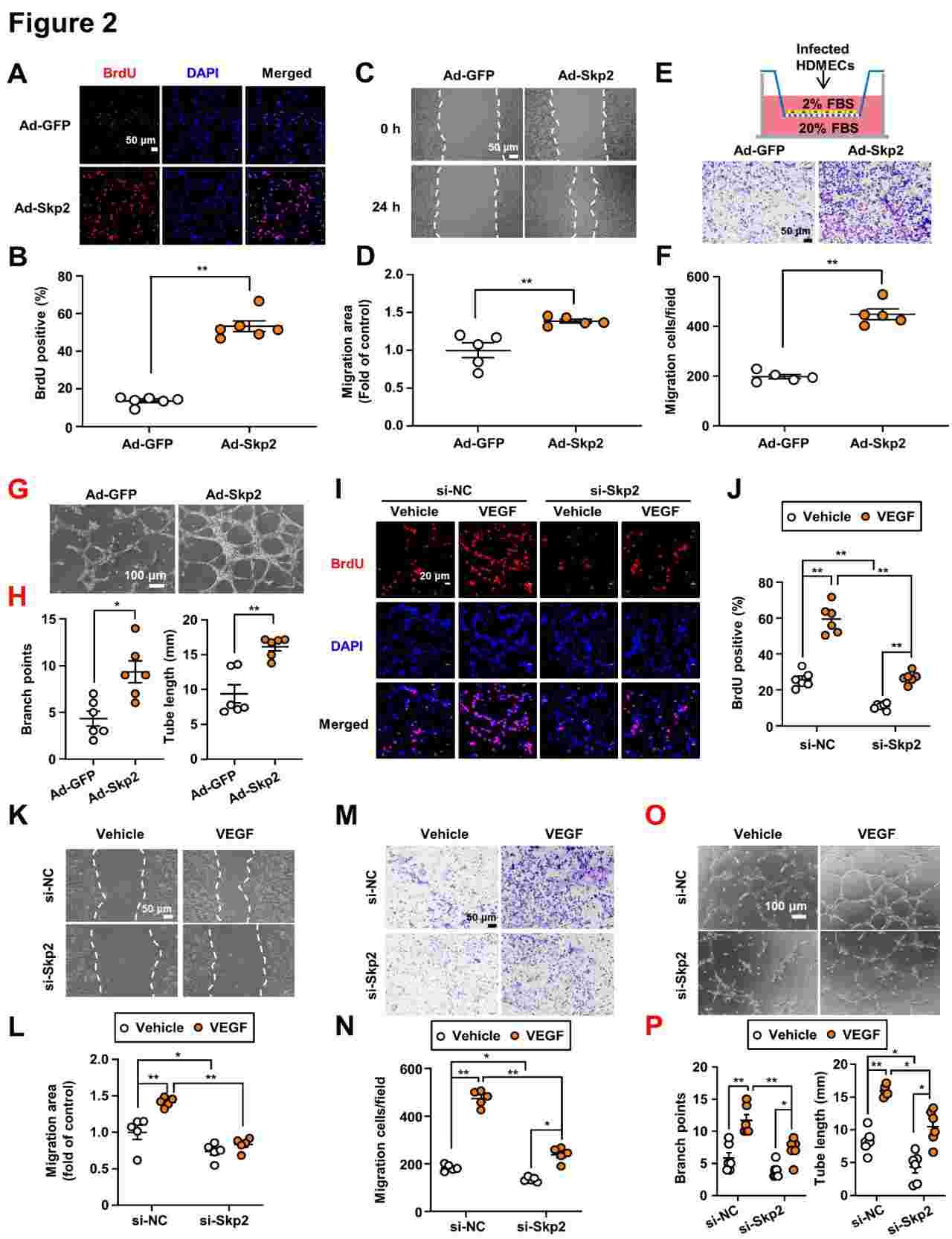 Fig. 2. Skp2 promoted angiogenesis in vitro and in vivo (Xie X, Cui Q, et al., 2024).
Fig. 2. Skp2 promoted angiogenesis in vitro and in vivo (Xie X, Cui Q, et al., 2024).
Ask a Question
Write your own review