- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The C17.2 cell line was derived from the cerebellum of neonatal C57BL/6 × CD-1 mice. Immortalisation was induced by retroviral transfer of the avian v-myc oncogene. Morphologically, they display a variable phenotype, either neuroblast-like or epithelioid. They maintain strong multipotency and can differentiate into both neurons and glia in vitro and in vivo, making them a useful system to study neurodevelopment, as well as neurogenesis and gliogenesis. In response to defined cues, the cells readily express neuronal markers such as β-III tubulin, as well as glial markers such as GFAP, faithfully recapitulating key steps of neural lineage progression.
As a result, C17.2 has been broadly used for neuroscience research, including studies of neural-stem-cell fate, neural repair and regeneration, as well as therapeutics for neurodegenerative diseases. For example, the line has been used to show that deletion of the Toe1 gene markedly suppresses proliferation and differentiation, and has also been used to study μ-opioid-receptor-mediated tolerance, as well as the effects of microglial polarisation on neural stem cells.
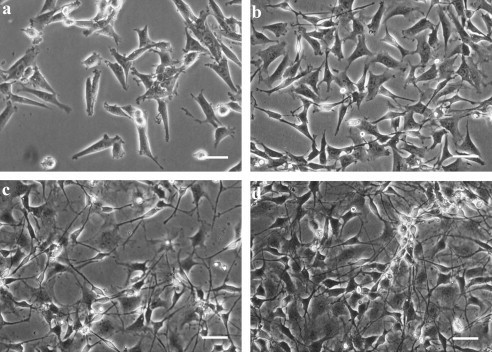 Fig. 1. Morphology of neural progenitor C17.2 cells and differentiated C17.2 cells (Lundqvist J, Andaloussi-Lilja J E, et al., 2012).
Fig. 1. Morphology of neural progenitor C17.2 cells and differentiated C17.2 cells (Lundqvist J, Andaloussi-Lilja J E, et al., 2012).
Viability of C17.2 Cells Treated with Curcumin and H2O2
Alzheimer's disease (AD) impairs adult neurogenesis partly through oxidative damage to neural stem cells (NSCs). While curcumin shows antioxidant and neuroprotective potential, its direct effect on NSCs under oxidative stress is unclear. Using H₂O₂-stressed C17.2 NSCs, Ruan et al. measured ROS, MDA, SOD activity, autophagy markers (LC3-II, Beclin-1) and p-ERK via Western blot.
The viability of C17.2 cells treated with varying concentrations of curcumin and H2O2 for specific time periods was assessed using CCK-8 kits. As depicted in Figure 1, curcumin exhibited no significant cytotoxic effect at the concentration up to 20 μM after a 24-h incubation period. In comparison to the cells treated with 10 μM curcumin, it demonstrated a neuroprotective effect after 6 h of incubation. These findings suggest that pre-treating cells with 10 μM curcumin for 24 h is sufficient to protect against H2O2-induced cell injury.
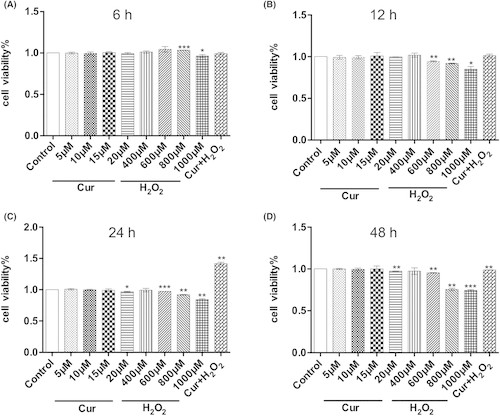 Fig. 1. Viability of C17.2 neural stem cells treated with curcumin (Cur), H2O2 or Cur combined with H2O2 (Ruan Y, Luo H Y, et al., 2024).
Fig. 1. Viability of C17.2 neural stem cells treated with curcumin (Cur), H2O2 or Cur combined with H2O2 (Ruan Y, Luo H Y, et al., 2024).
PNS Protected C17.2 Cells from OGD/R Damage
Stroke causes irreversible neural damage; endogenous neurogenesis is key to repair. Panax notoginseng saponins (PNS) previously enhanced NSC proliferation in vivo via mTOR. Here, Gao et al. asked whether PNS directly drives differentiation and neurite outgrowth of C17.2 NSCs after ischemic-like injury.
Initially, to determine the cytotoxic effect of PNS on C17.2 cells, we treated C17.2 cells with different concentrations of PNS for 24 hours. In Fig. 2B, the cell viability didn't show obvious cytotoxicity after PNS 6.25, 12.5, 25, 50, 100, 200, 400 ug/ml treatment compared with the control group. However, higher concentration of PNS 800 ug/ml showed a significant decrease in the cell viability. As shown in Fig. 2A and C, the cell viability of C17.2 cells in the control group was set as 100%. After C17.2 cells were subjected to OGD/R (2 h/24 h), the cell viability markedly dropped to 42.05±5.28%. Meanwhile, C17.2 cells displayed remarkable morphological changes, such as round, shriveled, deciduous and even dead cells. These results revealed that C17.2 cells were severely damaged after OGD/R, while PNS 50, 100 and 200 ug/ml presented the protective effect, showing the obviously increased cell viability than the OGD/R group. These results demonstrated that PNS could effectively protect C17.2 cells from OGD/R-induced injuries.
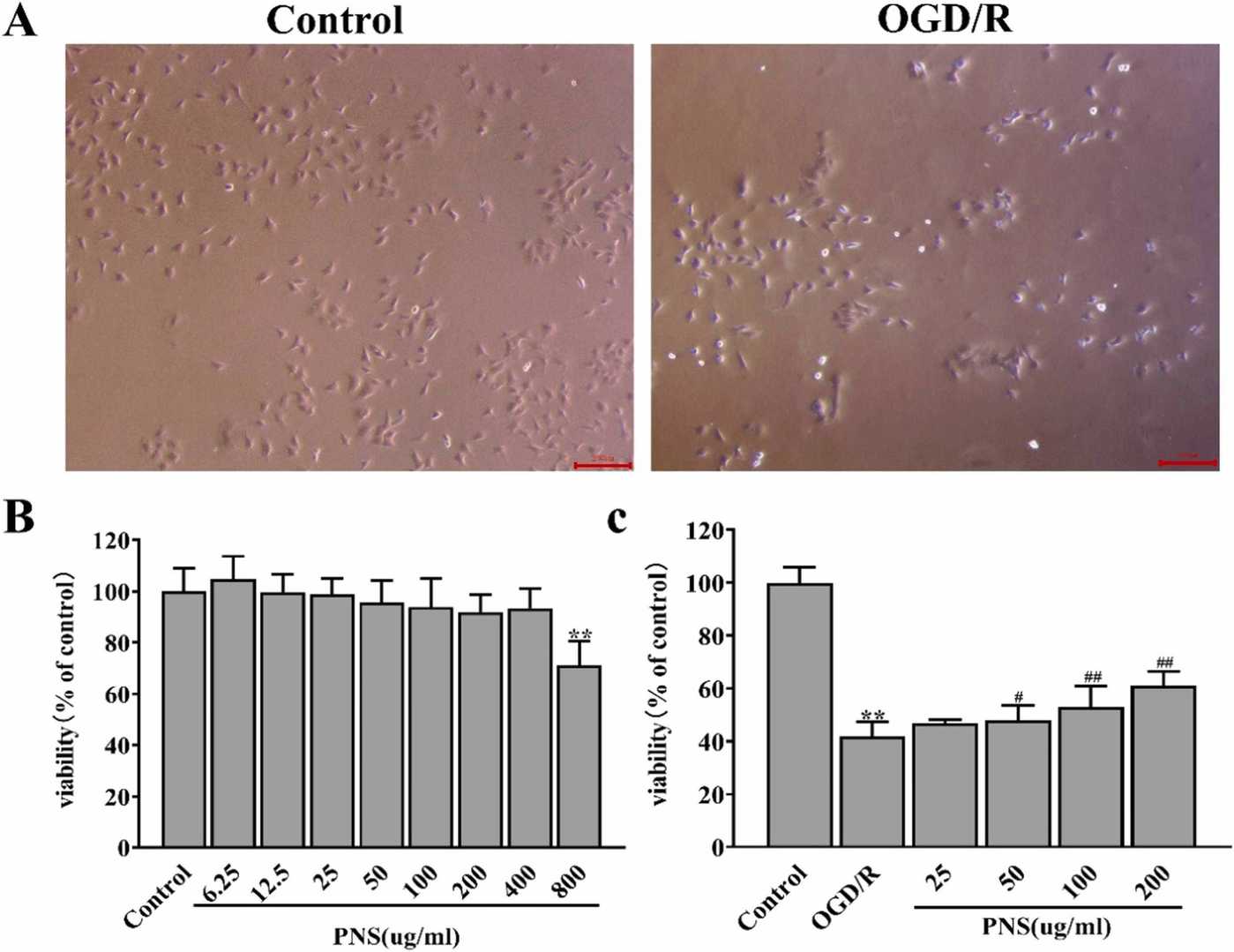 Fig. 2. The protective effect of PNS toward OGD/R-damaged C17.2 cells (Gao J L, Yao M J, et al., 2024).
Fig. 2. The protective effect of PNS toward OGD/R-damaged C17.2 cells (Gao J L, Yao M J, et al., 2024).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells