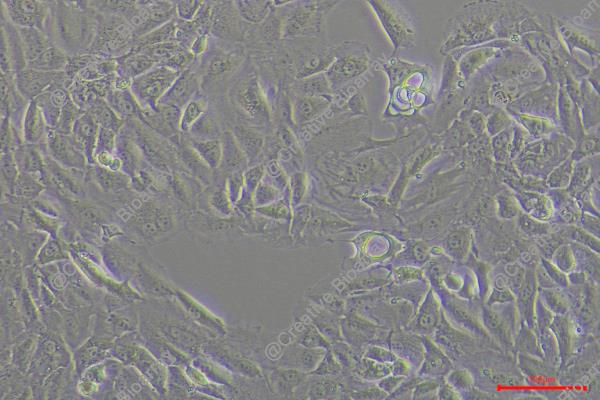
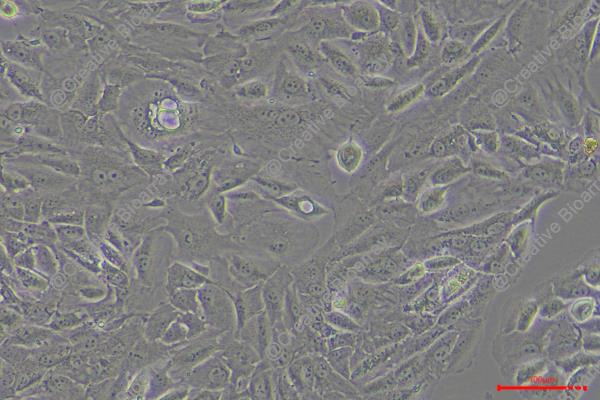
TUHR14TKB
Cat.No.: CSC-C6381J
Species: Homo sapiens (Human)
Morphology: epithelial-like
Culture Properties: Adherent cells
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Store in liquid nitrogen.
The TUHR14TKB cell line which develops from renal tubular epithelial cells belongs to renal cell carcinoma (RCC) and originated from a Japanese male patient. The TUHR14TKB cell line displays distinct genetic expression features by showing increased levels of the FABP7 gene and its transcription factor activity relative to other RCC cell lines. The raised expression levels of the EXOSC1 gene suggest its involvement in RNA degradation or gene regulation processes. In research applications, scientific studies utilize TUHR14TKB cells to examine gene activities related to RCC cellular growth, movement and DNA repair through genetic modification of FABP7, EXOSC1 and XRCC1 genes. This cell line also functions as a tool to test drug responses including promoter methylation regulation by 5-azacytidine in kidney cancer cells.
Silencing BCAR4 Gene Fusions Decreases Cell-Cycle Progression and Proliferation in Cancer Cells (TUHR14TKB and SNU308)
Chromosomal rearrangements in cancer genomes frequently lead to gene fusions, impacting oncogenesis and serving as crucial diagnostic and therapeutic markers. In this study, a comprehensive analysis across 9,638 patients revealed BCAR4 as the most prevalent gene fusion partner in solid tumors. Nixkless et al. aim to validate BCAR4's oncogenic role by examining its effect on cell proliferation through functional experiments in cancer cell lines.
Using RNA-seq data from the Cancer Cell Line Encyclopedia (CCLE) analyzed with INTEGRATE, they identified BCAR4 fusions in two cell lines: SNU308 gallbladder cancer cells (expressing LITAF-BCAR4) and TUHR14TKB renal carcinoma cells (expressing ZC3H7A-BCAR4). These lines, expressing BCAR4 fusions endogenously, are ideal to study their impact on cancer phenotypes. To explore if BCAR4 fusions drive cell-cycle progression, siRNAs transiently silenced the fusions, and flow cytometry assessed EdU incorporation and DNA content. Fusion knockdown significantly reduced S-phase cells and increased G1 phase cells (Fig. 1A and B). The siRNAs consistently decreased S-phase cells by 10%-25% and increased G1 phase cells by 5%-19%. Fusion knockdown did not affect cell viability as per annexin staining. These results demonstrate that endogenous BCAR4 fusions alter cell-cycle S-phase entry in these cancer lines.
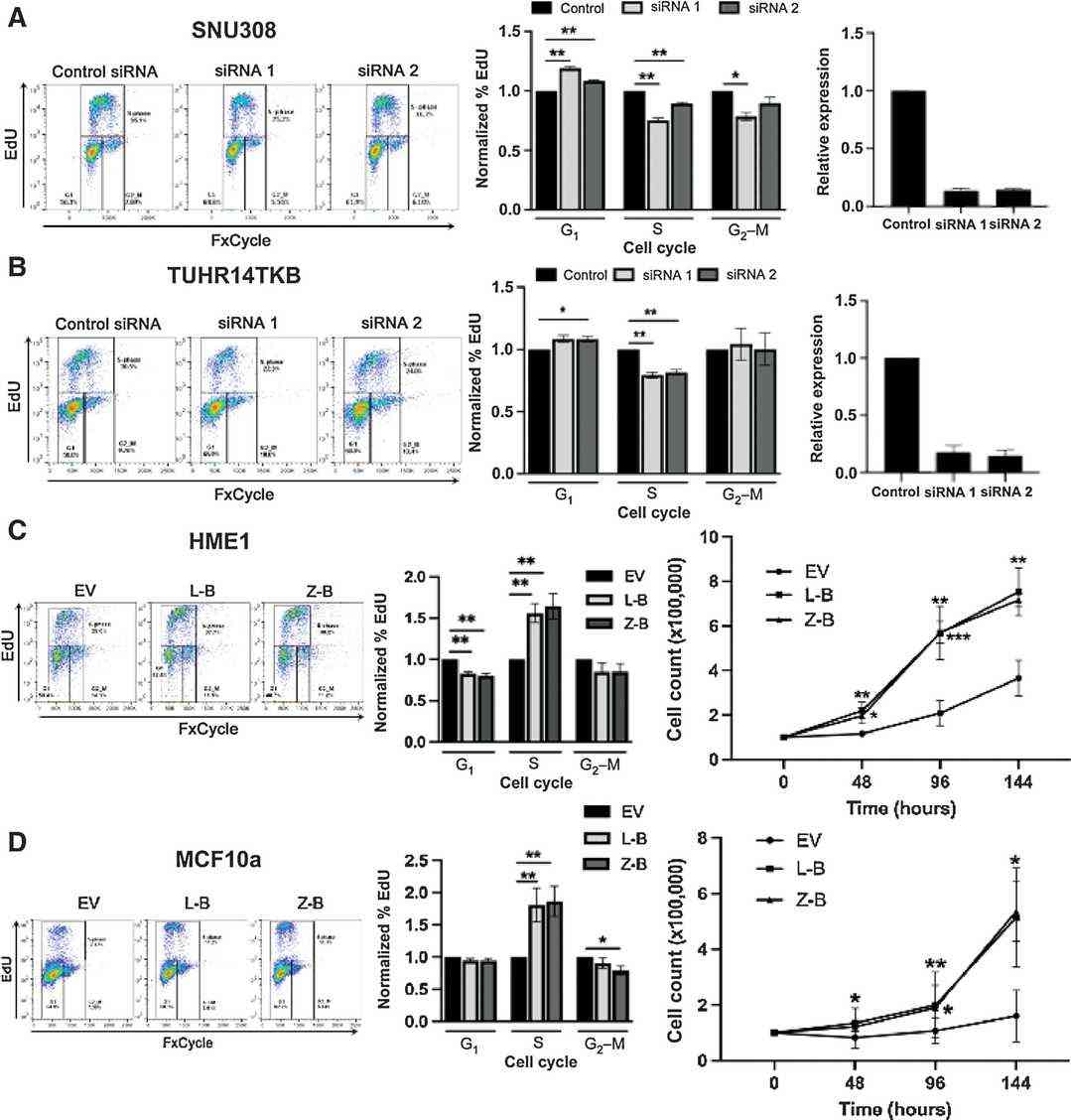 Fig. 1. BCAR4 gene fusions alter cell-cycle and proliferation (Nickless A, Zhang J, et al., 2022).
Fig. 1. BCAR4 gene fusions alter cell-cycle and proliferation (Nickless A, Zhang J, et al., 2022).
Functional Analysis of FABP7 in RCC Cells (TUHR14TKB)
Kidney cancer is a prevalent malignancy, with renal cell carcinomas (RCCs) constituting the majority. Identifying molecular markers like fatty acid binding protein 7 (FABP7) is critical for diagnosis and treatment. Takaoka et al. aim to clarify FABP7's role in RCC by analyzing its effects on cell proliferation and migration in relation to fatty acid composition.
They transfected FABP7 low-expressing TUHR14TKB and 786-O cells with an FABP7 expression vector (Fig. 2a and b; Fig. 3a and b). In 10% FBS, TUHR14TKB cells overexpressing FABP7 had a significantly longer doubling time than control vector cells (Fig. 4a, b). While control vector TUHR14TKB cells proliferated, FABP7-overexpressing cells did not in 1% FBS. TUHR14TKB FABP7 showed increased G2/M phase, indicating G2 arrest (Fig. 4c). In contrast, 786-O cell proliferation was stimulated by FABP7 overexpression in 1% FBS (Fig. 3c). Wound-healing assays showed TUHR14TKB FABP7 cells migrated slower than controls (Fig. 2c), while 786-O migration was unaffected (Fig. 3d).
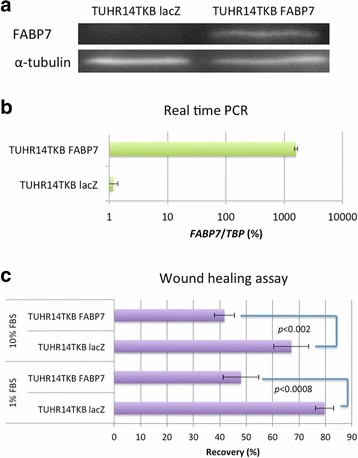 Fig. 2. Effect of FABP7 on the migration of TUHR14TKB cells (Takaoka N, Takayama T, et al., 2017).
Fig. 2. Effect of FABP7 on the migration of TUHR14TKB cells (Takaoka N, Takayama T, et al., 2017).
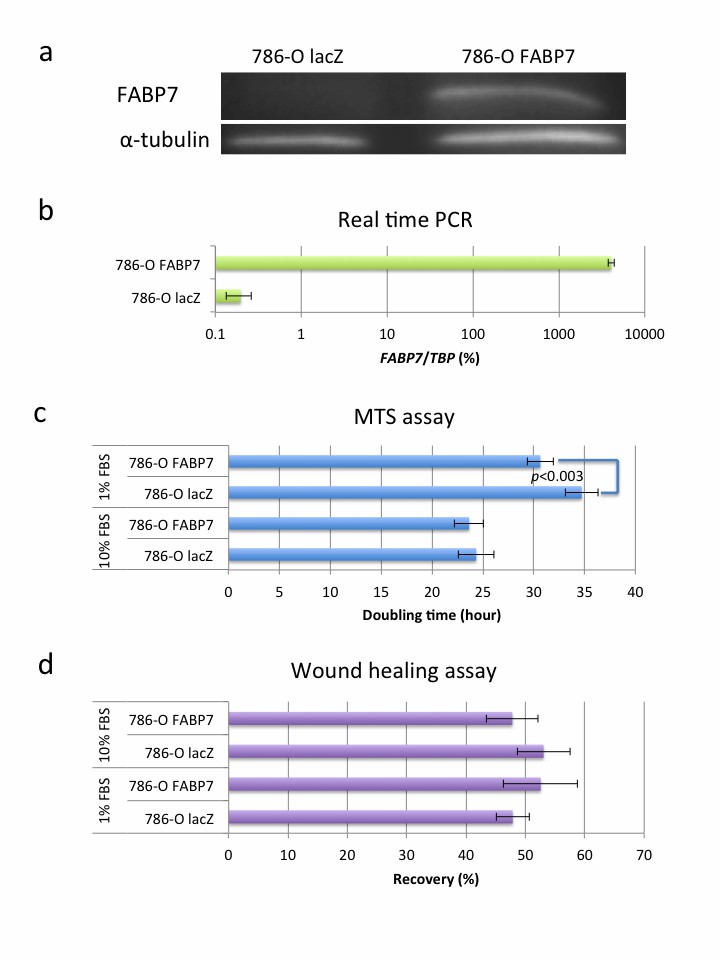 Fig. 3. Effect of FABP7 overexpression on the 786-O cell line (Takaoka N, Takayama T, et al., 2017).
Fig. 3. Effect of FABP7 overexpression on the 786-O cell line (Takaoka N, Takayama T, et al., 2017).
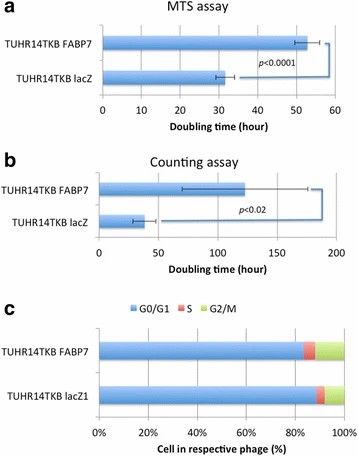 Fig. 4. Effect of FABP7 on cell proliferation and cell cycle of TUHR14TKB cells (Takaoka N, Takayama T, et al., 2017).
Fig. 4. Effect of FABP7 on cell proliferation and cell cycle of TUHR14TKB cells (Takaoka N, Takayama T, et al., 2017).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells