Mouse Synovial Fibroblasts
Cat.No.: CSC-C5387S
Species: Mouse
Source: Synovium
Cell Type: Fibroblast; Synoviocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse synovial fibroblasts from Creative Bioarray are isolated from the mouse joint tissue. The method we use to isolate mouse synovial fibroblasts was developed based on a combination of established and our proprietary methods. The mouse synovial fibroblasts are characterized by immunofluorescence with antibodies specific to vimentin. Each vial contains 0.5x10^6 cells per ml and is delivered frozen.
The primary source of mouse synovial fibroblasts (SFs) is the synovial tissue of joints specifically from the sublining layer. A thin tissue layer known as the synovium envelops the joint cavity and produces synovial fluid which lubricates the articular cartilage. Synovial fibroblasts function as key matrix-producing cells within synovial tissue while producing collagen, fibronectin, and hyaluronic acid to sustain articular cartilage structure and functionality. Under inflammatory conditions synovial fibroblasts release multiple inflammatory factors such as IL-1β, TNF-α and IL-6 together with cytokines RANKL, OPG and VEGF which promote inflammation and tissue damage. RANKL secretion from synovial fibroblasts activates osteoclast differentiation and activation processes that result in bone erosion and joint destruction.
In rheumatoid arthritis research synovial fibroblasts serve as key models and potential targets for genetic therapeutic approaches. Anti-inflammatory genes like IL-10 and TNFαR p55 when delivered into synovial fibroblasts through viral vectors help reduce their inflammation which leads to decreased joint damage. Researchers use synovial fibroblasts to study multiple signaling pathways which include the IHH-Gli, Erk1/2-ETS1, and P38 MAPK routes. Research findings show that the IHH-Gli signaling pathway drives collagen-induced arthritis rat synovial fibroblast proliferation.
Impact of Different Extracellular Na+ Concentrations on Synovial Fibroblasts
Millions of people suffer from osteoarthritis (OA) which generates significant healthcare expenses. The development of OA relies on mechanical stress due to its dual role in both protecting and harming joints based on its intensity. Various factors affect inflammation and bone remodeling processes and sodium levels serve as one of these significant influences. Proff et al. analyzed how murine synovial fibroblasts responded to different sodium chloride concentrations when subjected to mechanical strain.
They first examined the effects of varying extracellular salt levels on intracellular Na+ without mechanical loading. Lowering extracellular Na+ by 20 mM reduced intracellular Na+ (p = 0.03; Fig. 1a), while an increase of 50 mM raised it (p = 0.021). The transcription factor Nfat5 showed increased expression under high salt conditions (p ≤ 0.014; Fig. 1b). The transcription factor regulates the inflammatory genes Il6 and Ptgs2. High salt levels led to increased Il6 expression as indicated by p = 0.046 (Fig. 1c). The high salt environment significantly increased IL6 protein release as indicated by a p-value of 0.004 (Fig. 1d). Ptgs2 mRNA (p = 0.002; Fig. 1e) and PGE2 secretion (p < 0.001; Fig. 1f) also rose. High salt did not affect OPG at the mRNA (p ≤ 0.406; Fig. 1g) or protein level (p ≤ 0.509; Fig. 1h). However, RANKL mRNA expression (p = 0.013; Fig. 1i) and protein release (p = 0.013; Fig. 1j) increased. The increases in Il6/IL6, Ptgs2/PGE2, and Rankl/RANKL under high salt suggest that synovial fibroblasts may promote osteoclastogenesis.
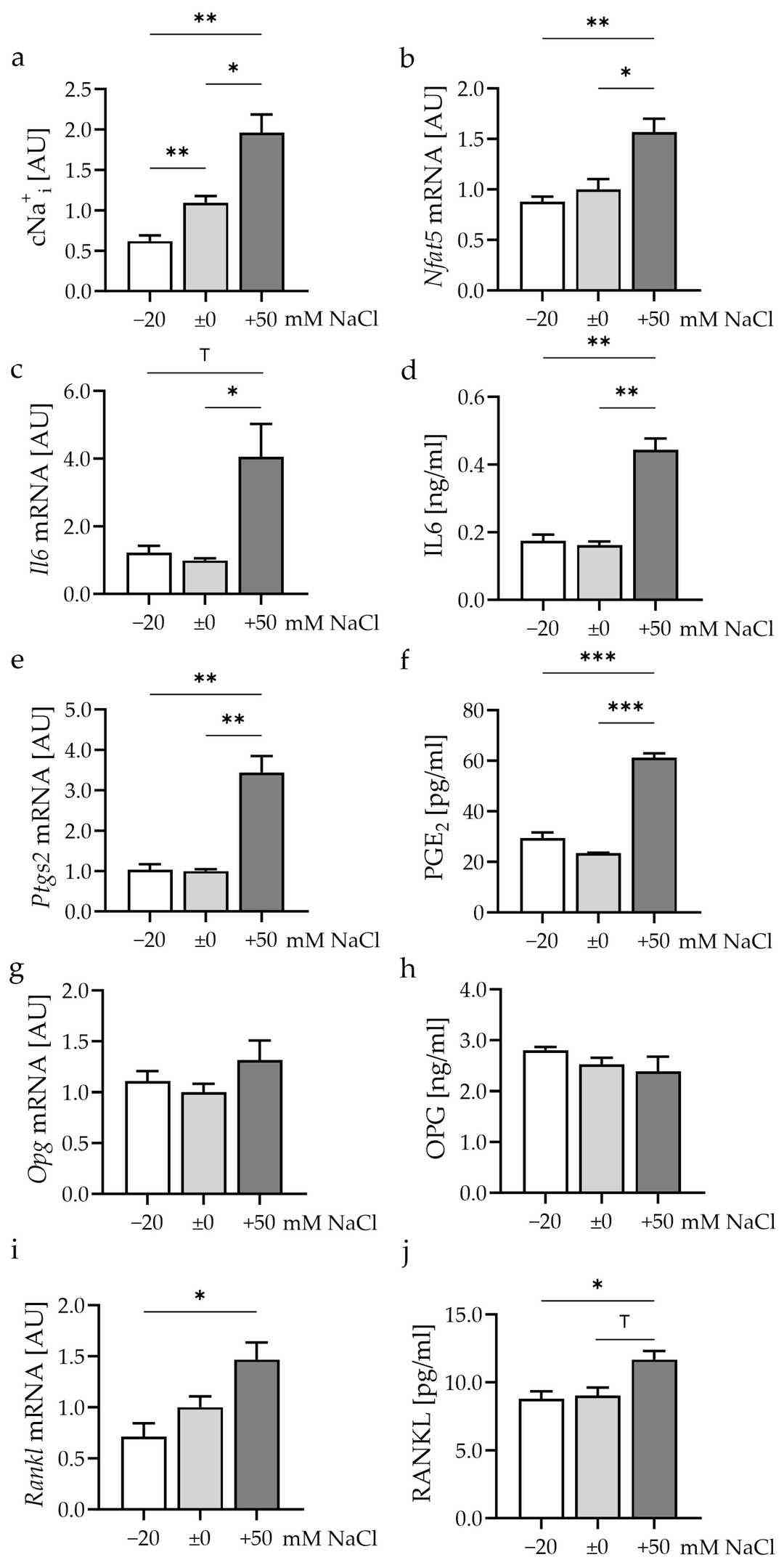 Fig. 1. Impact of different extracellular NaCl concentrations for 48 h (Proff A, Nazet U, et al., 2024).
Fig. 1. Impact of different extracellular NaCl concentrations for 48 h (Proff A, Nazet U, et al., 2024).
Effects Of GSH on the Mrna Levels of Pro-Infammatory Cytokines in LPS-Induced MH7A Cells and Mouse Sfs
Rheumatoid arthritis develops because of excessive reactive oxygen species which cause inflammation while encouraging synovial fibroblast growth. The depletion of glutathione (GSH), an essential antioxidant molecule, occurs frequently in RA patients which leads to increased oxidative stress while accelerating disease advancement. Hao's team examined GSH's potential to decrease ROS and inflammation in mouse synovial fibroblasts through PTEN/PI3K/AKT pathway modulation to uncover new RA treatment methods.
Mouse synovial fibroblasts were pretreated with different concentrations of glutathione (GSH) (50-150 μg/mL) for 24 hours to study its effects on cytokine production triggered by LPS in rheumatoid arthritis (RA) conditions. Figure 2A demonstrates that GSH at a concentration of 100 μg/mL reduced IL-6 secretion in cells stimulated by LPS (p<0.05), lowered TNF-α levels and elevated IL-10 mRNA concentrations (p<0.05). At a concentration of 100 μg/mL GSH IL-6 and TNF-α levels decreased while IL-10 levels increased in SFs as demonstrated by Figure 2B. MH7A cells showed decreased IL-6 and TNF-α levels upon treatment with GSH according to Figure 2c. The LPS+GSH group exhibited significantly reduced IL-1β mRNA levels compared to LPS alone in MH7A cells (p<0.05) which suggests GSH can modulate inflammatory pathways in RA.
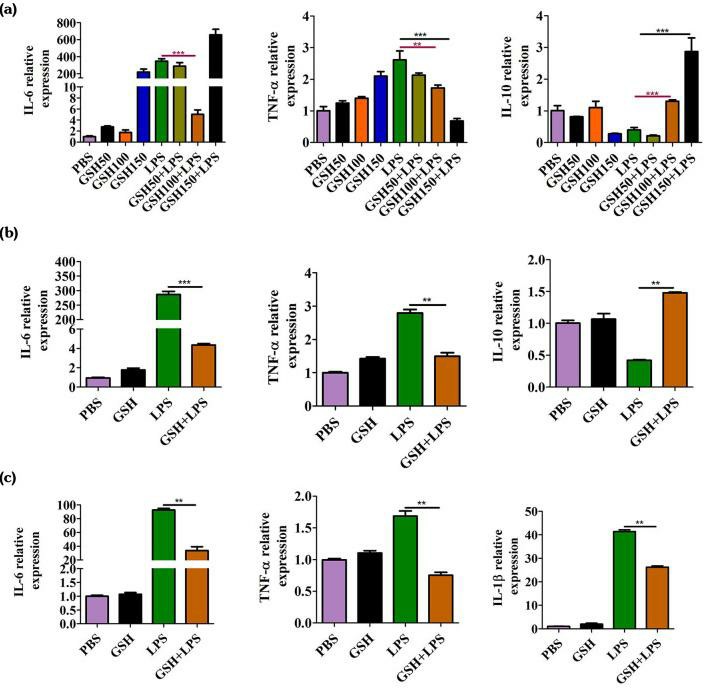 Fig. 2. The effects of reduced glutathione on the release of inflammatory cytokines (Hao WT, Huang L, et al., 2021).
Fig. 2. The effects of reduced glutathione on the release of inflammatory cytokines (Hao WT, Huang L, et al., 2021).
Ask a Question
Write your own review
- You May Also Need