IPEC-J2
Cat.No.: CSC-C2769
Species: Sus scrofa (Pig)
Source: Intestine; Small Intestine; Jejunum
Morphology: epitheloid cells growing adherently as monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Cell type: intestine (jejunum)
Origin: established from normal intestinal epithelium cells isolated from the jejunum of a neonatal, unsuckled pig
Helen Berschneider successfully isolated and cultured the IPEC-J2 cell line from neonatal pig jejunum tissue in 1989 at North Carolina State University. IPEC-J2 cell line maintains exceptional stability which supports infinite passage potential and emulates small intestinal epithelium physiology more accurately than colonic cell lines such as Caco-2, HT-29, and T84. Unlike these colonic lines, IPEC-J2 originates from the pig jejunum, providing a distinct advantage for studying porcine intestinal functions. It comprises a diverse array of cell phenotypes, mirroring the heterogeneity of intestinal epithelium. Functionally, IPEC-J2 cells excel in forming effective barrier functions with high transepithelial electrical resistance (TEER), especially when cultured with porcine serum (PS). They are capable of active transport activities, such as chloride secretion and ion transport, and possess strong immune responsiveness. These cells produce several immune-related molecules including MHC I, cytokines, chemokines, and mucins which help them detect pathogens such as Salmonella and pathogenic E. coli. The high metabolic activity of IPEC-J2 cells through glycolysis and oxidative phosphorylation among other pathways makes them an excellent model for studying intestinal biology research.
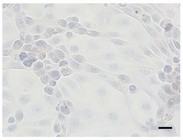 Fig. 1. IPEC-J2 cells immunohistochemically stained against Ki67 (Bockstal L V, Prims S, et al., 2024).
Fig. 1. IPEC-J2 cells immunohistochemically stained against Ki67 (Bockstal L V, Prims S, et al., 2024).
Toxic Effect of T-2 toxin on IPEC-J2 Cells
T-2 toxin, a prevalent mycotoxin in food and feed, poses significant risks to health through intestinal damage, which is not fully understood mechanistically. The MAPK signaling pathway, including pathways via JNK, ERK, and p38, regulates stress responses and inflammation. Using the IPEC-J2 cell line, Chen's team investigated whether T-2 toxin activates these pathways, particularly focusing on JNK, to induce oxidative stress and inflammation.
Using the CCK-8 method to examine the toxicity of T-2 toxin on IPEC-J2 cells, they first noted the impact of T-2 toxin on IPEC-J2 cell survival. In accordance with Fig. 1A, in contrast to the 0 ng/mL group, T-2 toxin had a substantial inhibitory impact on IPEC-J2 viability when the dose was more than 2 ng/mL. Therefore, T-2 toxin concentrations of 0, 1, 2, and 4 ng/mL were chosen for the follow-up studies. Further study showed that the LDH release significantly increased with the rose T-2 toxin treatment concentration (Fig. 1B). When IPEC-J2 cells were handle with T-2 toxin in contrast to untreated cells, the morphology changed, the intercellular space became larger, and the number decreased (Fig. 1C). These findings demonstrated that the T-2 toxin was toxic to IPEC-J2 cells.
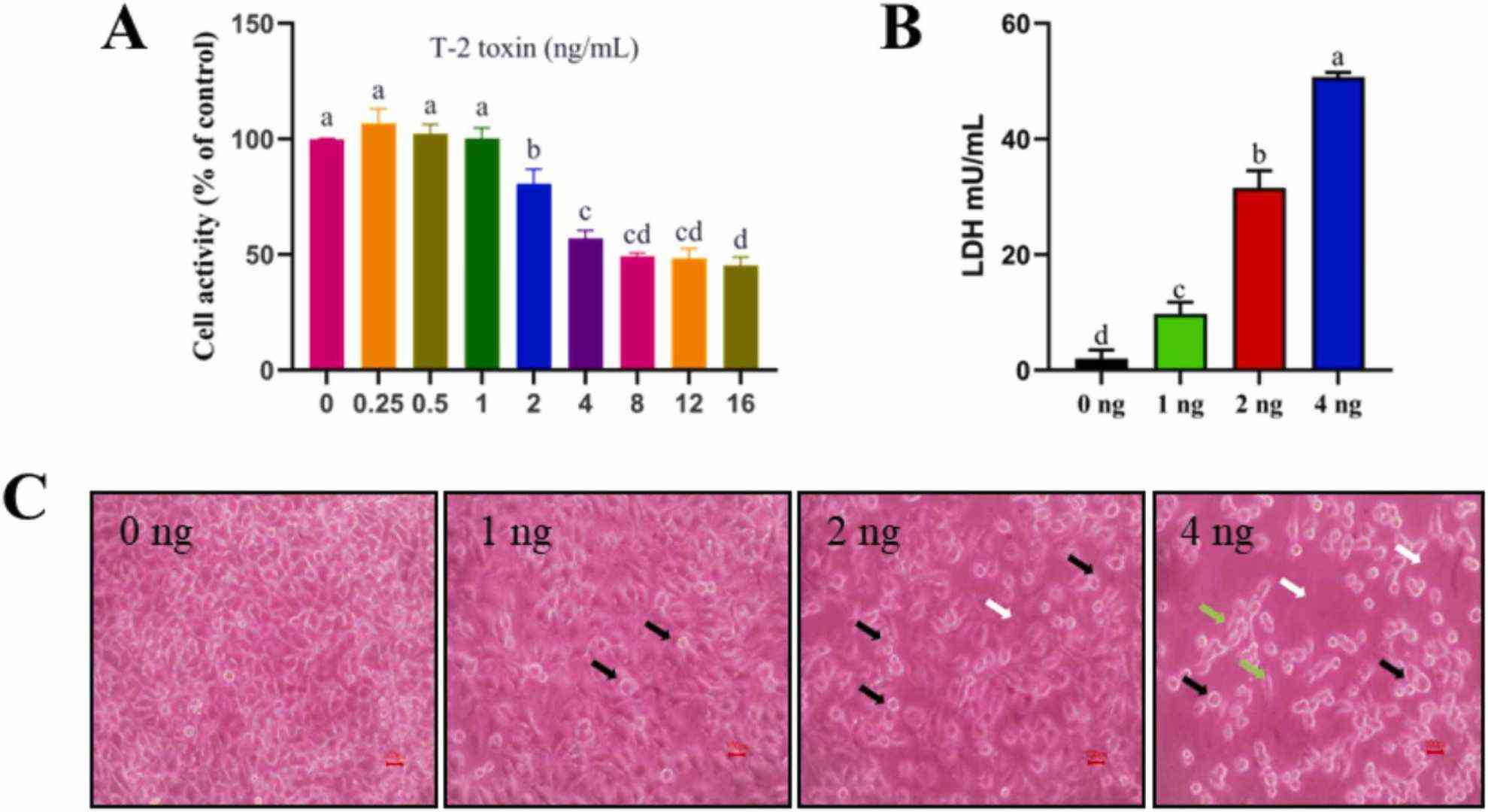 Fig. 1. Toxic effect of T-2 toxin on IPEC-J2 cells (Chen FJ, Wang YH, et al., 2023).
Fig. 1. Toxic effect of T-2 toxin on IPEC-J2 cells (Chen FJ, Wang YH, et al., 2023).
Protective Effect of Genistein on Cell Viability and Intracellular ROS Levels on IPEC-J2 cells
Interest in natural feed additives for animal health has increased, focusing on genistein, a soybean isoflavone aglycone with strong antioxidant properties. While promising for pig intestinal health, its mechanism against oxidative stress was unclear. Li's team used the hydrogen peroxide-stimulated IPEC-J2 cells oxidative stress model was employed to explore the antioxidant capacity of genistein and potential mechanisms.
As revealed in Figure 2, cell viability was significantly reduced from 100% to 69.8% in the H2O2-treated group compared to the control group. However, in contrast to the H2O2-treated group, pretreating cells with 10, 20, and 40 μM genistein before H2O2 exposure enhanced cell viability from 69.8% to 83.8%, 86.5%, and 83.2%, respectively. For subsequent experiments, we opted for a concentration of 20 μM genistein as it exhibited enhanced cell viability at this particular dosage. As demonstrated in Figure 3, a significant elevation in intracellular ROS levels was observed in the H2O2-treated group compared to the control group. In contrast to the H2O2-treated group, pretreating cells with 20 μM genistein before H2O2 exposure showed a tendency to reduce intracellular ROS levels. Comparatively, the genistein treated group did not have elevated intracellular ROS levels.
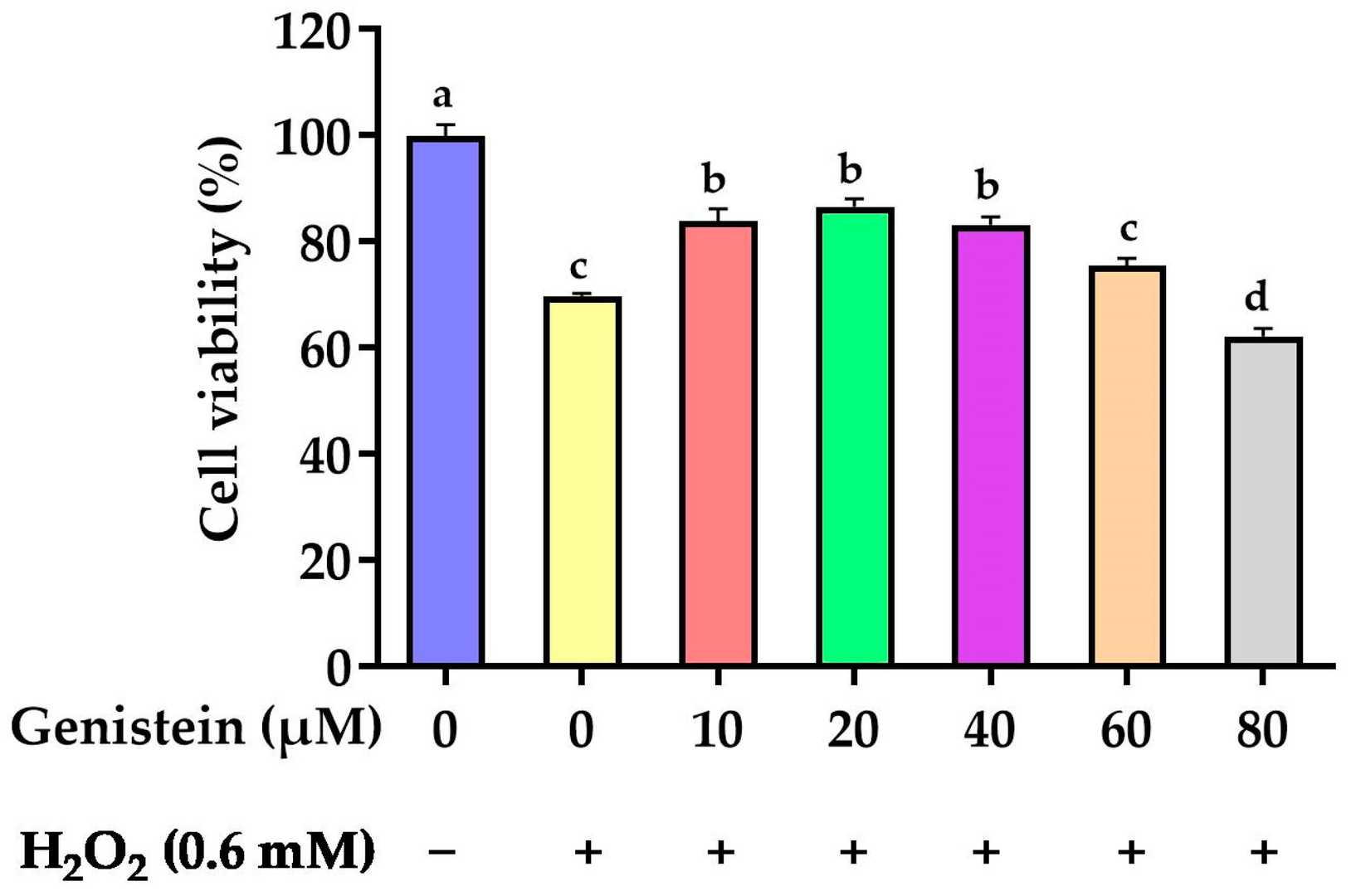 Fig. 2. Effect of genistein on the IPEC-J2 cells viability (Li Y, Cai L, et al., 2024).
Fig. 2. Effect of genistein on the IPEC-J2 cells viability (Li Y, Cai L, et al., 2024).
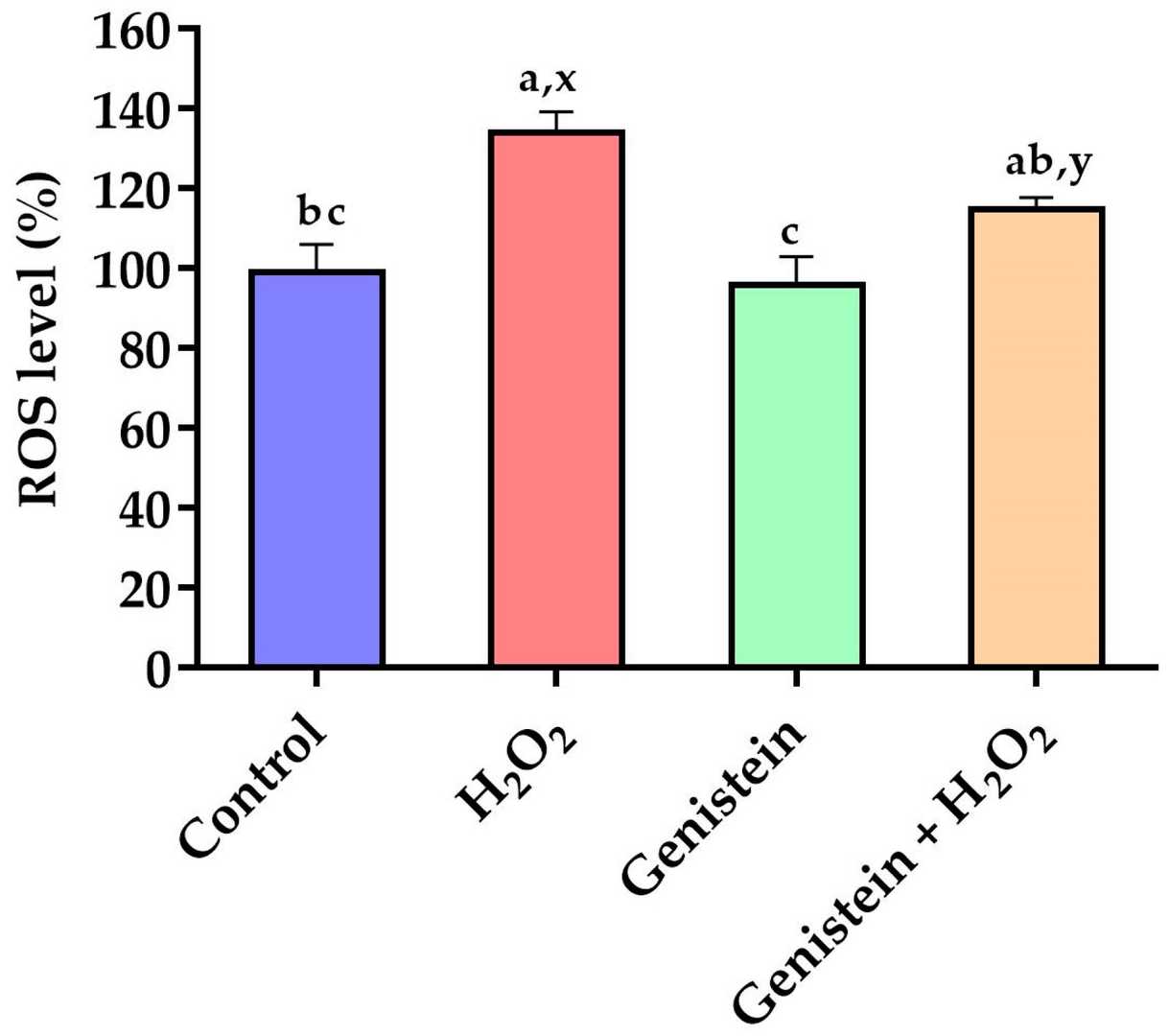 Fig. 3. Effect of genistein on ROS levels in IPEC-J2 cells (Li Y, Cai L, et al., 2024).
Fig. 3. Effect of genistein on ROS levels in IPEC-J2 cells (Li Y, Cai L, et al., 2024).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells