Human Mesenchymal Stem Cells
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human Mesenchymal Stem Cells (hMSCs) represent multipotent adult stem cells that can be found throughout different tissues and organs such as bone marrow, fat tissue, umbilical cord, placenta, and muscle. With the right in vitro or in vivo induction conditions hMSCs can transform into diverse cell types including osteoblasts, chondrocytes, adipocytes, neurons, hepatocytes and cardiomyocytes. The ability of hMSCs to differentiate into multiple cell types positions them as valuable tools for tissue repair and regenerative medicine.
hMSCs demonstrate immunomodulatory functions that enable them to suppress inflammatory responses and control immune cell activity. The distinctive properties of hMSCs enable them to provide special benefits in autoimmune disease treatment and transplant rejection reduction. hMSCs show standard surface markers CD73, CD90, and CD105 but do not display hematopoietic markers CD45 and CD34. The low immunogenicity of hMSCs helps prevent immune system rejection during allogeneic transplantation procedures. Furthermore, hMSCs demonstrate a significant ability to multiply quickly which enables their rapid growth in laboratory conditions to produce a sufficient quantity of cells for medical treatments. Therefore, hMSCs serve as essential cell lines for use in cell therapy treatments.
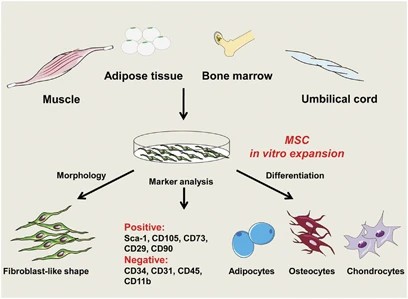 Fig. 1. The properties of MSCs (Ma S, Xie N, et al., 2014).
Fig. 1. The properties of MSCs (Ma S, Xie N, et al., 2014).
Effects of MXene NPs on Calcium Deposition and Mineralization of hMSCs
Bone tissue damage remains a significant challenge in dental and orthopedic therapy due to limitations of current treatments. Lee et al. developed a bioink incorporating MXene nanoparticles into GelMA and HAMA to explore its potential in bone tissue engineering. The bioink was 3D printed with encapsulated human mesenchymal stem cells (hMSCs) to evaluate its cytocompatibility and osteogenic properties.
To explore whether MXene nanoparticles (NPs) can induce osteogenic differentiation of human mesenchymal stem cells (hMSCs), they conducted ARS and von Kossa staining to measure calcium deposition. Calcium deposition is a key marker of late-stage osteogenesis. ARS staining showed no significant calcium deposition at 7 days for cells treated with up to 40 μg/mL MXene NPs compared to controls. However, significant increases were observed at 14 and 21 days, with 20 μg/mL MXene NPs yielding the highest deposition (Fig. 1a and b). Von Kossa staining also revealed increased mineralized bone nodules at 21 and 28 days in hMSCs treated with MXene NPs, compared to controls (Fig. 1c and d). Previous studies suggest that MXene NPs below 20 μg/mL can upregulate early and late osteogenic genes, promoting differentiation. Based on cytotoxicity and osteogenesis assessments, we determined the optimal MXene NP concentration to be 20 μg/mL.
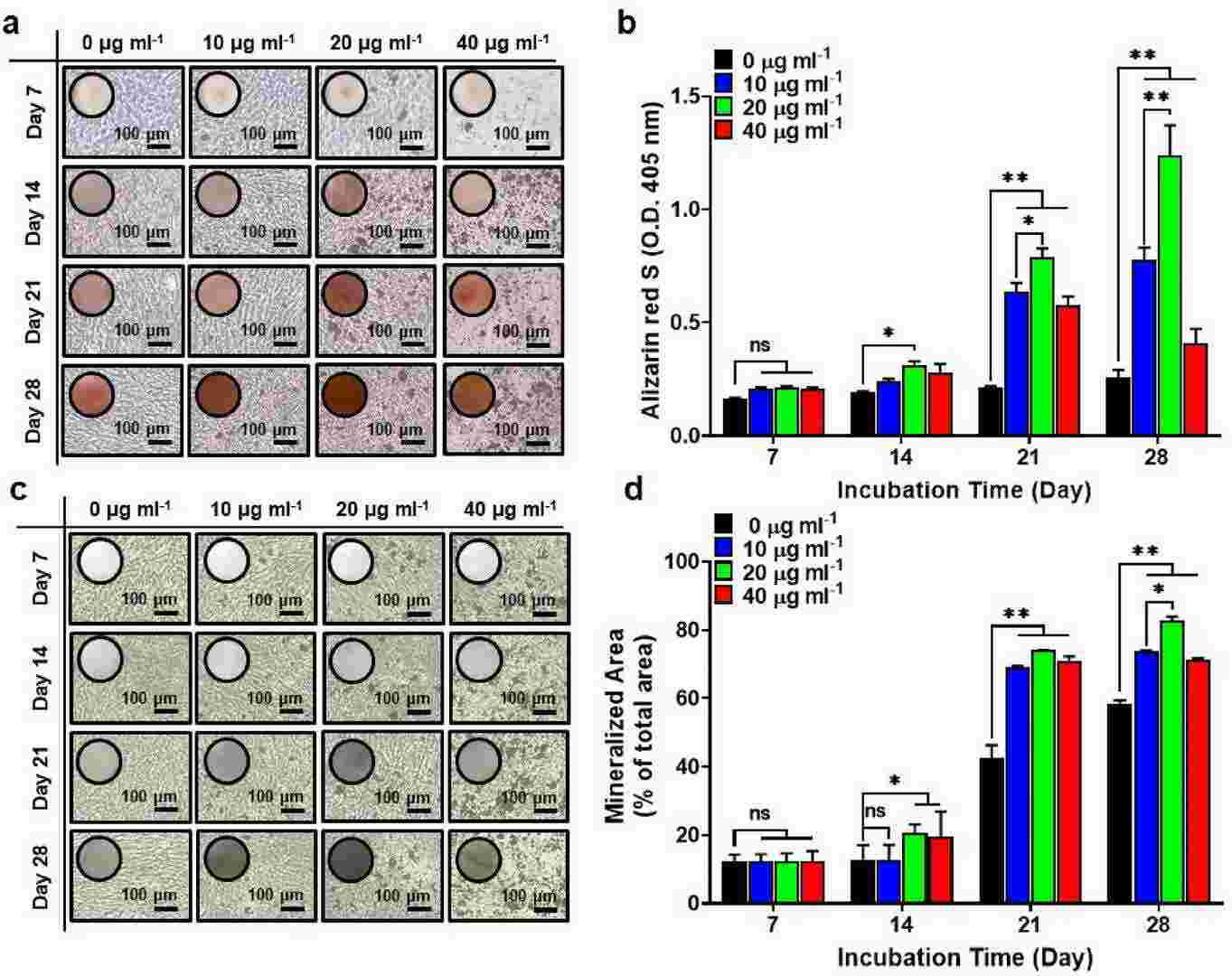 Fig. 1. Osteogenic differentiation of hMSCs treated with MXene NPs (Lee S H, Kang M S, et al., 2023).
Fig. 1. Osteogenic differentiation of hMSCs treated with MXene NPs (Lee S H, Kang M S, et al., 2023).
Higher Viscosity of Extracellular Fluid Enhanced Cell Spreading
Human mesenchymal stem cells (hMSCs) could serve as a treatment for osteoporosis but their osteogenic differentiation requires further improvement. While viscosity affects cellular functions through biomechanical signals the specific influence of viscosity on hMSC osteogenesis remains unknown. Chen's team studied the biomechanical behavior of hMSCs by growing them in a high-viscosity medium to examine changes in cellular spreading and intracellular tension.
They studied how ECF viscosity affects hMSCs' biomechanical properties and differentiation by changing the culture medium's viscosity using different methylcellulose (MC) concentrations (0%, 0.2%, 0.4%, 0.8%). Viscosity increased with more MC, with values at 0%, 0.2%, 0.4%, and 0.8% being 0.98 ± 0.04, 8.44 ± 1.94, 21.83 ± 3.21, and 68.14 ± 1.59 cP, respectively (Fig. 2A and B). Viscosities at 0.4% and 0.8% MC are similar to hMSCs' natural environments like bone marrow. They tested medium viscosity effects on biomechanical properties, including cell spreading, actin polymerization, and focal adhesion. hMSCs were cultured on glass and observed with IRM, showing rapid spreading in high-viscosity medium (Fig. 2C), which also increased cell spread (Fig. 2D and E). They further analyzed viscosity impact over six hours, showing cell spread peaked at 60 minutes and fluctuated due to migration.
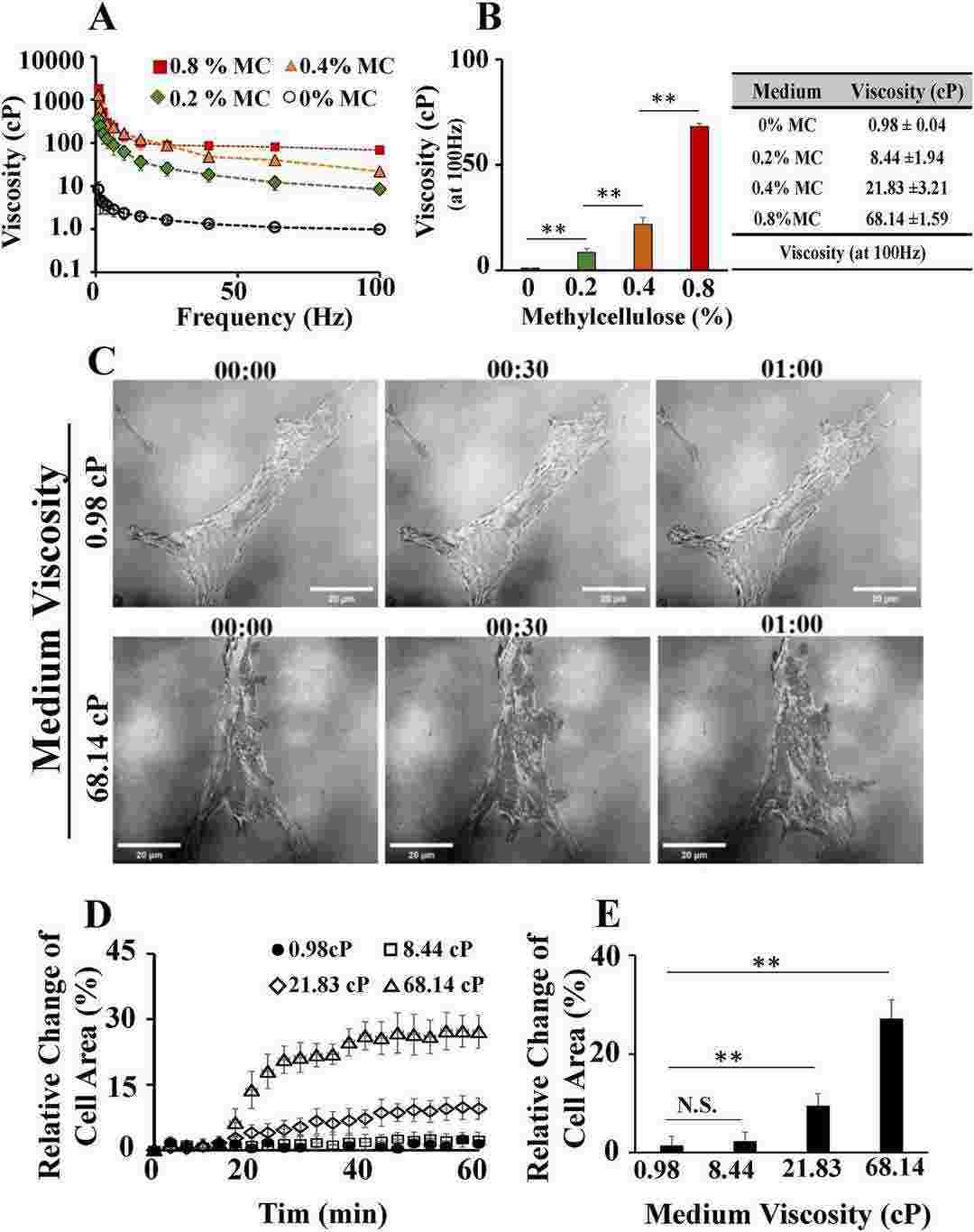 Fig. 2. Higher viscosity of extracellular fluid significantly increased the spreading area of human mesenchymal stem cells (hMSCs) (Chen YQ, Wu MC, et al., 2024).
Fig. 2. Higher viscosity of extracellular fluid significantly increased the spreading area of human mesenchymal stem cells (hMSCs) (Chen YQ, Wu MC, et al., 2024).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells