Hs 578T
Cat.No.: CSC-C9433L
Species: Homo sapiens (Human)
Source: Breast
Morphology: epithelial
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Tumorigenecity: yes, in nude mice
Isoenzyme: G6PD, B; PGM3, 1; PGM1, 1; ES-D, 1; Me-2, 0; AK-1, 1; GLO-1, 1
Histopathology: carcinoma, ductal
vWA: 17
FGA: 23,24
Amelogenin: X
TH01: 9,9.3
TPOX: 8
CSF1P0: 13
D5S818: 11
D13S317: 11
D7S820: 10
The Hs 578T cell line is a human breast cancer cell line derived from a carcinoma of the mammary gland. These cells exhibit an epithelial-like morphology and are characterized by their adherent growth pattern. The Hs 578T cell line is widely used in cancer research, particularly to study the mechanisms of breast cancer progression and metastasis. The cells display mutations in the TP53 gene, a key tumor suppressor gene that is often associated with the aggressive behavior of certain cancer types.
The Hs 578T cells are hormone receptor-negative, meaning they do not express estrogen or progesterone receptors, which classifies them as triple-negative breast cancer cells. This makes them particularly valuable in the study of treatments targeting aggressive subtype of breast cancer, which typically has fewer therapeutic options and a poorer prognosis compared to hormone receptor-positive breast cancers. Researchers have utilized the Hs 578T cell line to explore various aspects of tumor biology, including cell proliferation, migration, and response to chemotherapy and targeted therapies.
Hs 578T cell line also expresses vimentin, a marker associated with epithelial-to-mesenchymal transition (EMT), a process that plays a crucial role in cancer metastasis. Studies involving these cells help to elucidate the molecular pathways involved in EMT and provide insights into potential therapeutic targets for inhibiting cancer spread. Additionally, Hs 578T cells have been used in drug screening assays to identify compounds with potential anticancer activity.
LAF Inhibited the Migration and Invasion of TNBC Cells by Inhibition of EMT Features
Migration and invasion are hallmarks of malignant tumors, with EMT serving as a crucial early stage in the malignant transformation of tumor cells. This process facilitates the detachment of tumor cells from the primary tissue and their entry into the circulatory system. Drugs that target the EMT process could potentially inhibit the systematic metastasis of carcinomas, thereby prolonging patient survival.
Lappaol F (LAF), a lignan extracted from Fructus Arctii, has a wide spectrum of anti-tumor effects, including inhibition of Triple-negative breast cancer (TNBC) cell growth. Effects of LAF on the migration and invasion capabilities of TNBC cells were assessed via wound healing assay and Transwell assay after 24 h, respectively. The wound healing assay (Fig. 1A-D) demonstrated that incubation with 25 or 50 μM LAF for 24 h significantly inhibited wound healing in both cell lines. Concurrently, the Transwell assay (Fig. 1E-G) indicated that the addition of 10, 25, or 50 μM LAF markedly reduced the number of tumor cells in the lower chamber. The results suggested that LAF directly prevents the migration and invasion of MDA-MB-231 and Hs-578T cells.
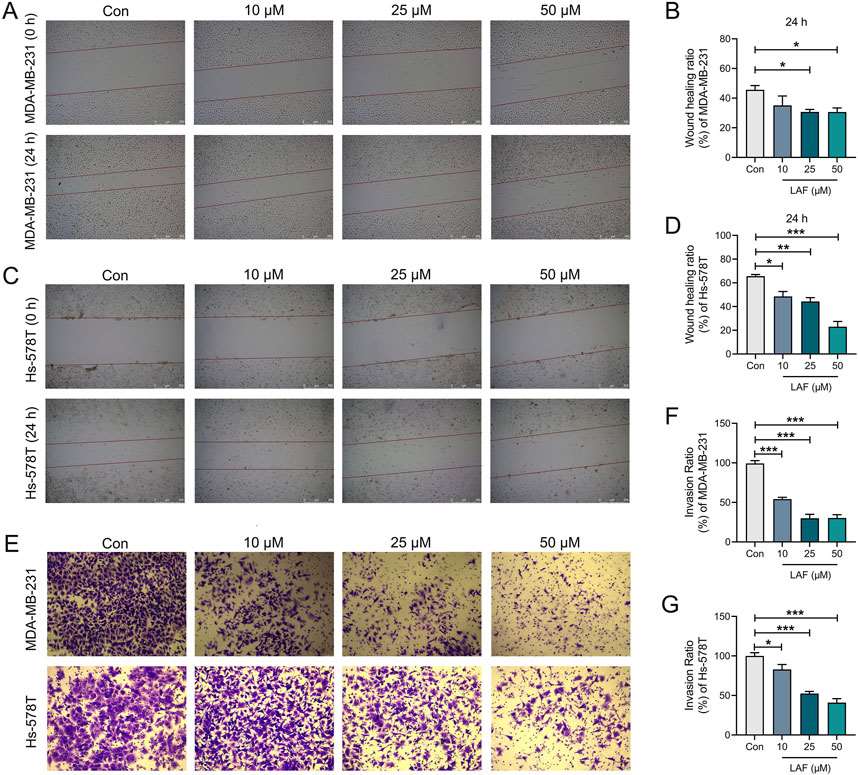 Fig. 1. Effects of LAF on the migration and invasion of TNBC cells (Meng, Qiqi, et al. 2025).
Fig. 1. Effects of LAF on the migration and invasion of TNBC cells (Meng, Qiqi, et al. 2025).
TNBC Cells Response to NCT-503 Treatment
This study aimed to investigate the impact of de novo serine biosynthetic pathway (SSP) inhibition, specifically targeting phosphoglycerate dehydrogenase (PHGDH) with NCT-503, on three TNBC cell lines: MDA-MB-231, MDA-MB-468 and Hs 578T.
The cytotoxicity potential of NCT-503 on the three TNBC cell lines was evaluated using the MTT assay. A 48h exposure design was utilized, with the compound tested across a concentration range of 0.48 to 250 μM. The NCT-503 treatment presented antiproliferative effects, expressed in a dose-dependent manner on all three TNBC cell lines (Fig. 2A). Both the IC20 and IC50 doses were used to evaluate the morphological changes induced by the NCT-503 treatment in the TNBC cell lines (Fig. 2B). Proteins significantly modified after NCT-503 treatment were determined using univariate analysis by comparing the protein abundance levels of treated samples to their respective controls (p ≤ 0.05, |FC| ≥ 1.2) (Fig. 2C).
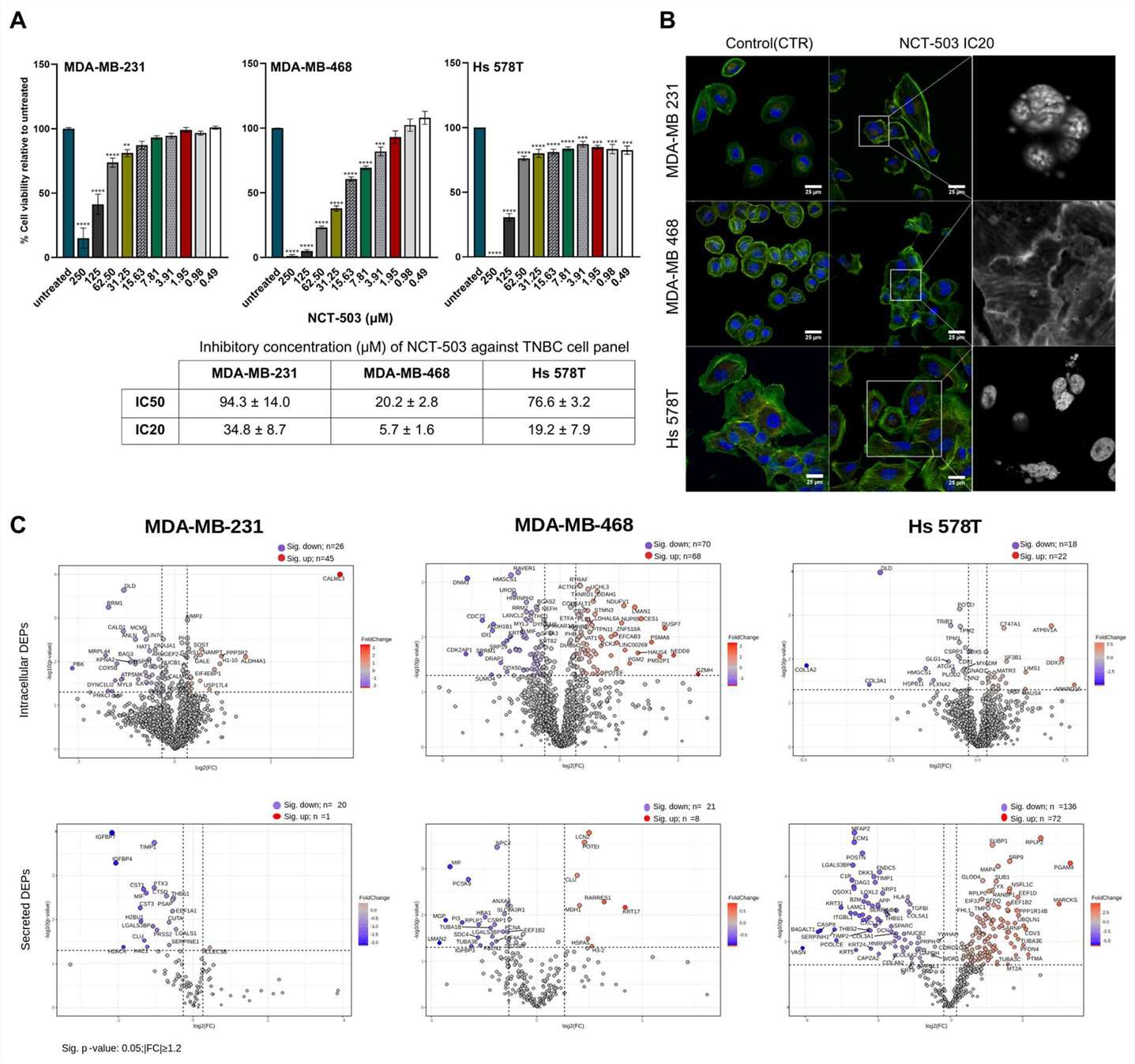 Fig. 2. In vitro evaluation of NCT-503 antiproliferative effect against TNBC cell panel (Pralea, Ioana-Ecaterina, et al. 2024).
Fig. 2. In vitro evaluation of NCT-503 antiproliferative effect against TNBC cell panel (Pralea, Ioana-Ecaterina, et al. 2024).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells