DEL
Cat.No.: CSC-C0397
Species: Homo sapiens (Human)
Source: Pleural Effusion
Morphology: grows often in clusters in suspension, most cells are polygonal (some cells are round)
Culture Properties: suspension
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: CD3 -, CD7 -, CD10 -, CD14 -, CD15 +, CD19 -, CD25 +, CD30 +, CD34 -
Viruses: ELISA: reverse transcriptase negative; PCR: EBV -, HBV -, HCV -, HHV-8 -, HIV -,
DEL cell line is derived from the pleural effusion of a 12-year-old boy with malignant histiocytosis in 1987 (possibly anaplastic large cell lymphoma, ALCL). This cell line tends to grow in suspension in culture. This DEL cell line contains the NPM1-ALK (NPM-ALK) fusion gene that was created by a chromosomal translocation, which is a common genetic disorder of ALCL in children and adults. The NPM1 (nucleophosmin) and ALK (anaplastic lymphoma kinase) proteins fusion into an oncogenic fusion protein. NPM-ALK drives ALCL pathogenesis by triggering various signaling pathways including JAK/STAT and PI3K/AKT/mTOR which are important for cell growth, survival and other oncogenes. For example, NPM-ALK positive tumor cells require ongoing activation of the JAK3/STAT3 pathway to continue to exist.
With the NPM-ALK fusion gene, DEL cell line is an invaluable resource for understanding ALCL pathology, disease progression and therapeutic targets. Scientists use this cell line for high-throughput drug testing and functional genetic analysis to discover inhibitors that block the NPM-ALK fusion protein that might be therapeutically useful for ALCL. In addition, genetic studies could explain the oncogenic activity of NPM1-ALK. Moreover, the DEL cell line can be used to study ALCL cellular biology, drug tolerance, and relationships with other cell lines.
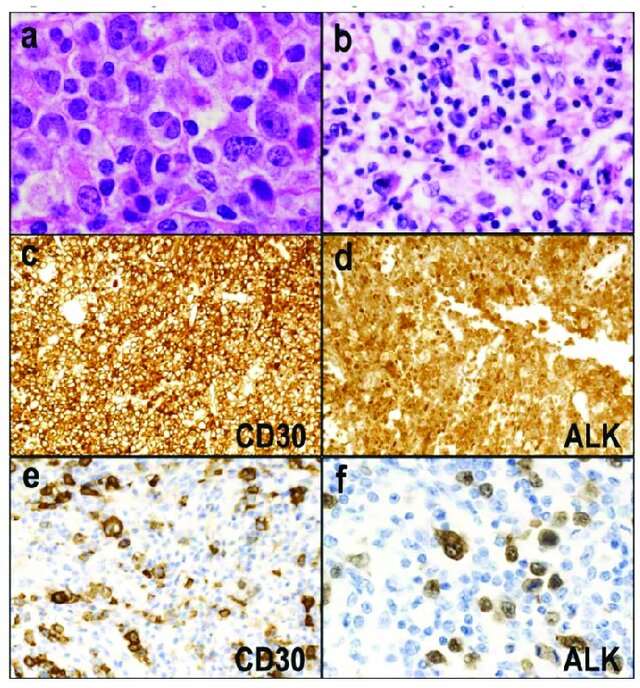 Fig. 1. ALK-positive anaplastic large cell lymphoma (ALCL) (Satou, A., Takahara, T., et al., 2022).
Fig. 1. ALK-positive anaplastic large cell lymphoma (ALCL) (Satou, A., Takahara, T., et al., 2022).
SQLE Deficiency and Cholesterol Auxotrophy in ALK+ ALCLs
This study explores the cholesterol dependency of cancer cells, particularly in ALK+ anaplastic large cell lymphoma (ALCL) cell lines (ALK+ ALCLs). Garcia-Bermudez et al. discovered that these cells rely heavily on cholesterol uptake due to a loss of squalene monooxygenase (SQLE), identifying the low-density lipoprotein receptor (LDLR) as crucial for their growth, presenting a potential therapeutic target. Using gene expression data from the cancer cell line encyclopedia (CCLE), Garcia-Bermudez et al. identified nine cell lines lacking SQLE mRNA and protein. Remarkably, six of these belong to the ALK+ ALCLs: DEL, SU-DHL-1, KIJK, SUP-M2, SR-786, and Karpas 299 (Fig. 1d and e). Similar to SNU-1, these SQLE-deficient ALCL cell lines are sensitive to cholesterol depletion and accumulate high levels of squalene (Fig. 1g and f). SRS microscopy revealed that ALK+ALCLs accumulate squalene in lipid droplets (Fig. 1h). The loss of SQLE expression is linked to promoter hypermethylation, with DNA-hypomethylating agents upregulating SQLE mRNA. Primary tumor samples and patient-derived xenografts of ALK+ ALCLs also showed reduced SQLE expression. Despite the inverse correlation between NPM-ALK translocation and SQLE expression, ALK signaling modulation did not affect SQLE expression, suggesting a different silencing mechanism (Fig. 2). These findings indicate that ALK+ ALCLs lack SQLE expression, making them cholesterol auxotrophs.
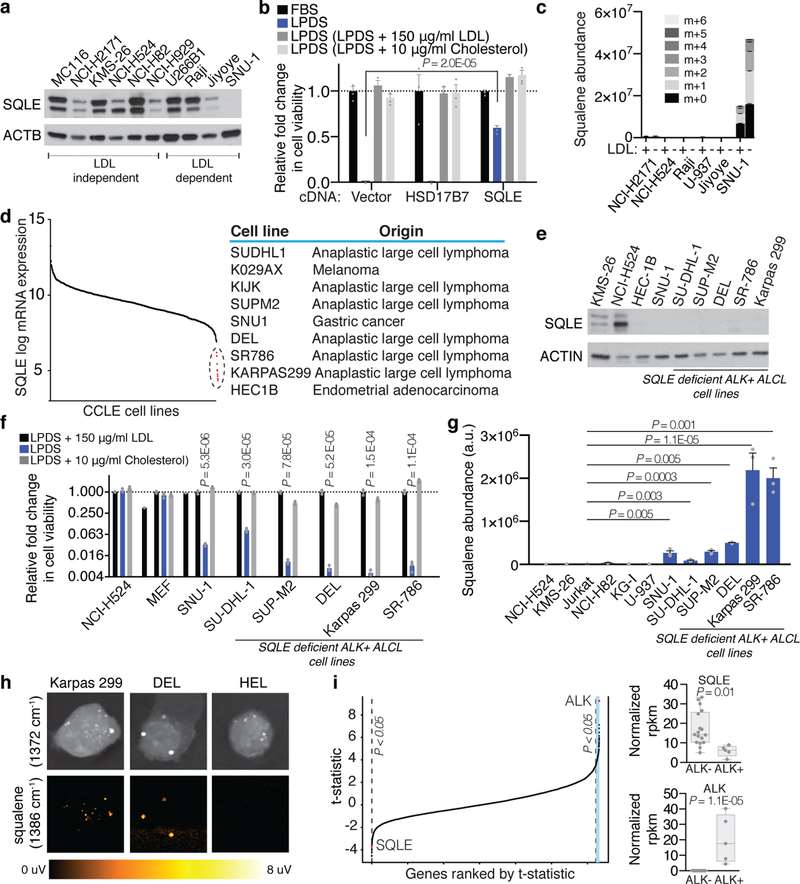 Fig. 1. ALK+ ALCLs are auxotrophic for cholesterol due to lack of SQLE expression (Garcia-Bermudez, J., Baudrier, L., et al., 2019).
Fig. 1. ALK+ ALCLs are auxotrophic for cholesterol due to lack of SQLE expression (Garcia-Bermudez, J., Baudrier, L., et al., 2019).
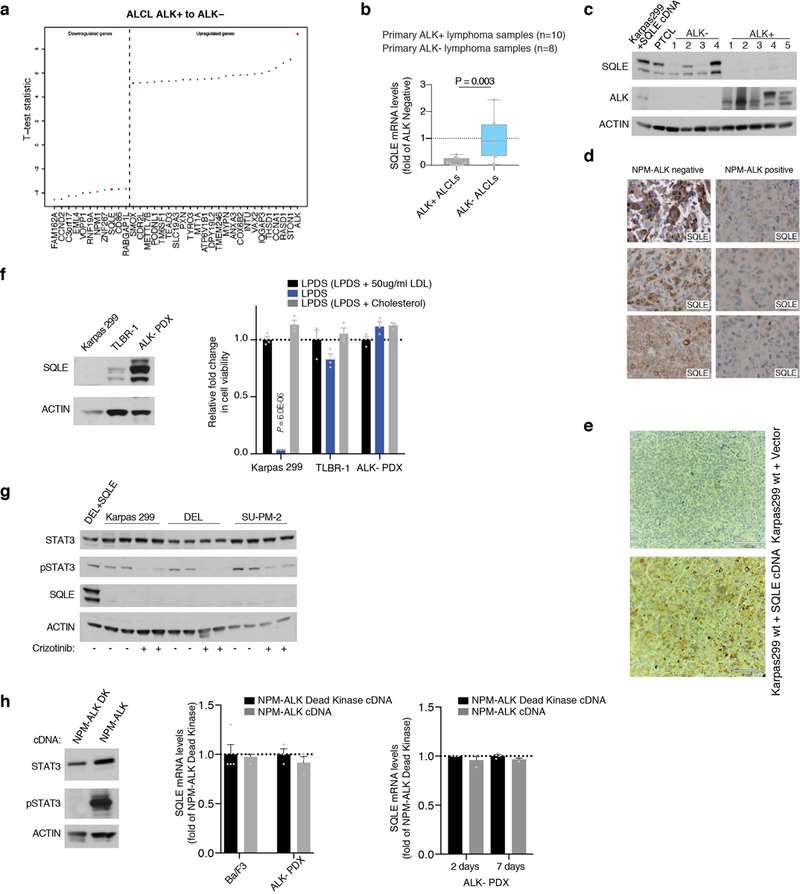 Fig. 2. Lack of SQLE expression in primary ALK+ ALCLs (Garcia-Bermudez, J., Baudrier, L., et al., 2019).
Fig. 2. Lack of SQLE expression in primary ALK+ ALCLs (Garcia-Bermudez, J., Baudrier, L., et al., 2019).
ALK Inhibitor Crizotinib Reduces ACLY Y682 Phosphorylation in ALK+ Lymphoma and Neuroblastoma Cells
Rapid cell proliferation requires metabolic adaptation for biomass synthesis, with ATP citrate lyase (ACLY) playing a crucial role in linking carbohydrate and lipid metabolism. Basappa J et al. investigated the role of tyrosine phosphorylation at Y682 in enhancing ACLY activity and its oncogenic implications in multiple cancers. Phosphoproteomics analysis of lymphoma cell lines with NPM-ALK fusion tyrosine kinase identified 881 phosphorylated tyrosine peptides. Key findings indicate that ACLY Y682 phosphorylation significantly correlates with ALK Y1604 activation. ALK inhibition via CEP-26939 reduced ACLY Y682 phosphorylation, confirmed by Crizotinib (an ALK inhibitor) treatment (Fig. 3f). They treated two ALK+ ALCL cell lines (SUDHL-1, DEL) and one neuroblastoma cell line (NB1) with Crizotinib, observing reduced Y682 phosphorylation through anti-pACLY Y682 antibody immunoblotting (Fig. 3a). Immunohistochemistry on biopsies from ALK+ and ALK- ALCL patients showed a strong correlation between ACLY Y682 phosphorylation and ALK expression (Fig. 3b). Creating an ALK+ ALCL cell line (DEL) with either an empty vector, HA-tagged ACLY-WT, or ACLY-Y682F, immunoblotting revealed increased Y682 phosphorylation in ACLY-WT but not in ACLY-Y682F cells (Fig. 3c). Additionally, transducing primary human T cells with either active NPM-ALK or kinase-deficient NPM-ALK-K210R showed ACLY Y682 phosphorylation only in cells with active NPM-ALK (Fig. 3e).
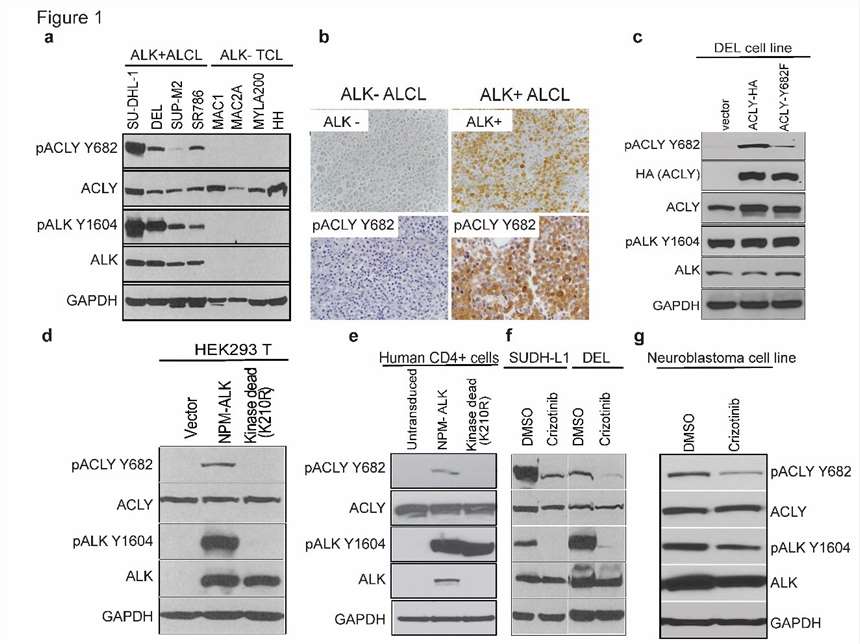 Fig. 3. NPM-ALK interacts with ACLY and phosphorylates on Y682 residue in ALK+ALCLs (Basappa J., ElAzzouny, M. A., et al., 2020).
Fig. 3. NPM-ALK interacts with ACLY and phosphorylates on Y682 residue in ALK+ALCLs (Basappa J., ElAzzouny, M. A., et al., 2020).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells