Monkey Bone Marrow Mesenchymal Stem Cells
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The primary source of Creative Bioarray's monkey BMSCs is bone marrow tissue extracted from cynomolgus monkeys. Mammalian bone marrow acts as the primary source for hematopoietic stem cells while also housing mesenchymal stem cells (MSCs) within its stromal cell population. Studies demonstrate that mesenchymal stem cells located in bone marrow play a dual role in sustaining hematopoietic system balance and providing support and regulation functions for hematopoietic stem cells. BMSCs have the ability to differentiate into multiple cell types including osteoblasts adipocytes and chondrocytes due to their multipotent differentiation capabilities. Research findings demonstrate that BMSCs show potential to transform into neuronal cells, endothelial cells, and hepatocytes when exposed to particular induction conditions. Moreover, BMSCs function as immune regulators by secreting cytokines which control immune responses and suppress inflammation in autoimmune diseases and tissue repair processes.
BMSCs show great potential for future therapies in regenerative medicine and cellular transplantation treatments. Bone tissue repair investigations alongside nerve regeneration studies and cartilage repair applications have utilized them. Researchers use monkey BMSCs to build 3D bioprinted scaffolds which support bone defect repair. Gene therapy research uses these cells to increase their therapeutic effectiveness by applying genetic modifications.
H2O2-induced hGC Ageing, and BMMSCs Reversed hGC Ageing
The aging of ovaries triggers endocrine system disruptions and tissue deterioration which drastically affects female health. Effective treatments are urgently needed. Earlier research demonstrated that bone marrow mesenchymal stem cells (BMMSCs) from juvenile rhesus monkeys enhanced ovarian structure and functionality in older rhesus monkeys. Tian et al. continue to discover how BMMSCs restore the youthful state of granulosa cells (GCs).
H2O2 functions as a small-molecule oxidant to trigger cell ageing through oxidative stress and it is commonly employed for this purpose. In this study, human GCs (hGCs) were exposed to H2O2 for 24 h and then cocultured with BMMSCs for 48 h. Under the fluorescence inverted microscope, model group GCs appeared flat and wide with many intracellular vacuoles and fewer cells compared to the control group. The coculture with BMMSCs led to improved GC morphology and higher cell numbers while reducing the number of vacuoles (Fig. 1A). The β-Galactosidase staining test revealed blue-stained hGCs in 7.33 ± 1.69% of control samples while 93.33 ± 0.47% of model group samples and 43.66 ± 2.05% of coculture group samples obtained blue staining (Fig. 1B and C). Immunohistochemical analysis of P53 protein expression levels showed 21.04 ± 0.48% in control cells while the model group displayed 58.20 ± 1.21% and the coculture group exhibited 42.90 ± 1.41% expression (Fig. 1D, E). BrdU staining showed that hGCs proliferation and division occurred with red-stained cells found at 87.66 ± 1.24% in the control group, 16.33 ± 1.24% in the model group and 80.66 ± 1.24% in the coculture group (Fig. 1F, G). ROS levels measured by DHE staining were 3.77 ± 0.12% in control group cells while model group cells showed 8.63 ± 0.12% and cells in the coculture group demonstrated 5.63 ± 0.12% (Fig. 1H, I). These results suggest that hGCs aged after 24 h of 273 mM H2O2 treatment, and BMMSCs reversed the ageing markers in aged hGCs.
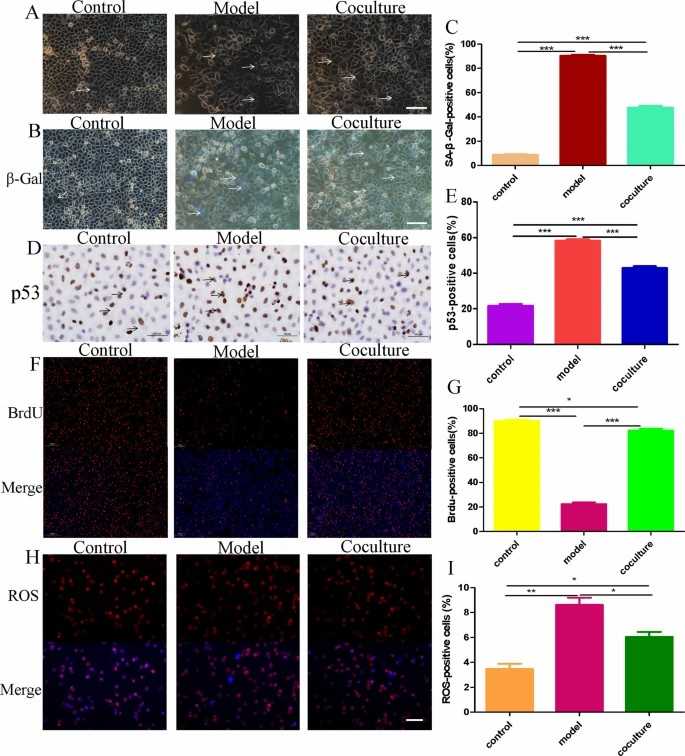 Fig. 1. hGC ageing model and coculture with BMMSCs (Tian C, An YY, et al., 2023).
Fig. 1. hGC ageing model and coculture with BMMSCs (Tian C, An YY, et al., 2023).
In Vitro Cytotoxicity of ZnO/Ag-Haw in Monkey Bone Marrow Mesenchymal Stem Cells
Hydroxyapatite (HA) is a commonly used bone repair material, but its antibacterial properties are insufficient for preventing infections. Ion doping and surface modification are potential solutions. In this study, silver ion-doped and ZnO nanoparticle-coated hydroxyapatite whiskers (ZnO/Ag-HAw) were prepared. Monkey bone marrow mesenchymal stem cells (MSCs) were used for test in vitro cytotoxicity of ZnO/Ag-Haw. Figure 2 shows the results of cytotoxicity of the samples by CCK-8. All samples have no cytotoxicity, and the lowest cell survival rate is 90. It can also be concluded that the presence of zinc ions stimulates cell proliferation and improves biocompatibility. Doping HA with zinc ions could increase cell viability and promote osseointegration.
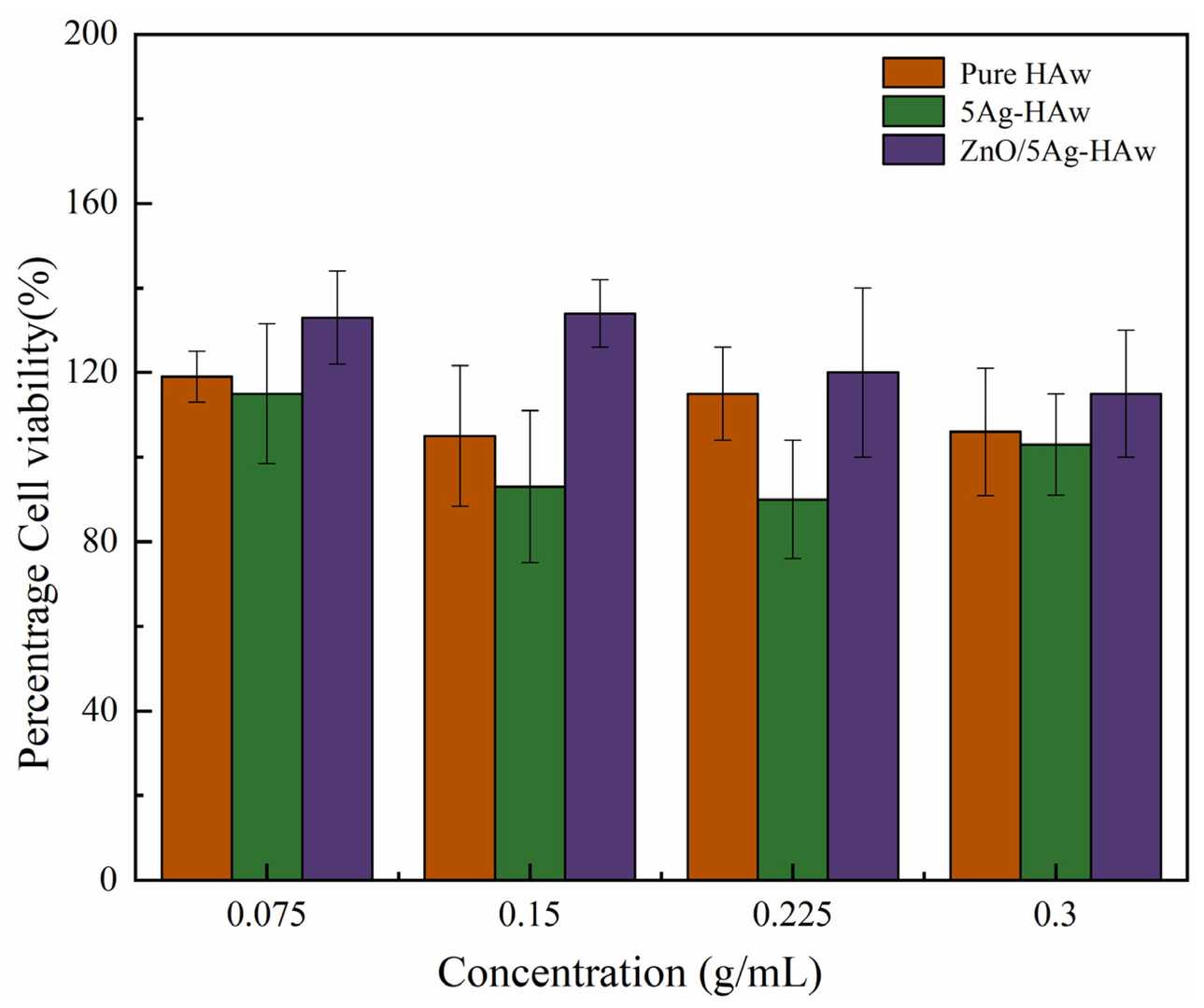 Fig. 2. Results of the CCK-8 assay (Yan T, Jiang Z, et al., 2021).
Fig. 2. Results of the CCK-8 assay (Yan T, Jiang Z, et al., 2021).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells