Human iPSC-derived Brain Microvascular Endothelial Cells
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human iPSC-derived Brain Microvascular Endothelial Cells (iBMECs) are derived from integration-free induced pluripotent stem cell (iPSC) lines under a fully defined proprietary induction condition. iBMEC cells are compact cellular structures when plated as a monolayer in culture and express standard biomarkers such as ECadherin and ZO-1 using immunohistochemistry methods and transporter biomarkers such as ABCB1 (P-gp), ABCG2, ABCE1, HIF1A, CLDN5, GLUT1, LAT1, MCT1, TJP1, PDGFbeta, OCLN, SLC25A3, TFR1, and SLC25A5 using RT/qPCR methods.
Human iPSC-derived Brain Microvascular Endothelial Cells (iBMECs) are brain microvascular endothelial cells (BMECs) differentiated from induced pluripotent stem cells (iPSCs). iPSCs are derived from adult cells (fibroblasts and blood cells) using genetic reprogramming methods (Sendai virus-mediated non-integrative reprogramming). iBMECs in vitro have typical endothelial cell morphology and characteristics, including cell-cell junctions, tight junctions, and adherens junctions. Under in vitro culture conditions, iBMECs typically form a monolayer with high cell density and tight cell-cell contacts. In a microfluidic system, iBMECs can form vascular-like structures with morphological and functional features of the in vivo blood-brain barrier structure. The characteristics of iBMECs allow this cell line to be used to measure drug penetration across the blood-brain barrier, as well as for drug metabolism and drug toxicity tests. Vascularized tissues and organ-on-a-chip models can be also constructed with iBMECs.
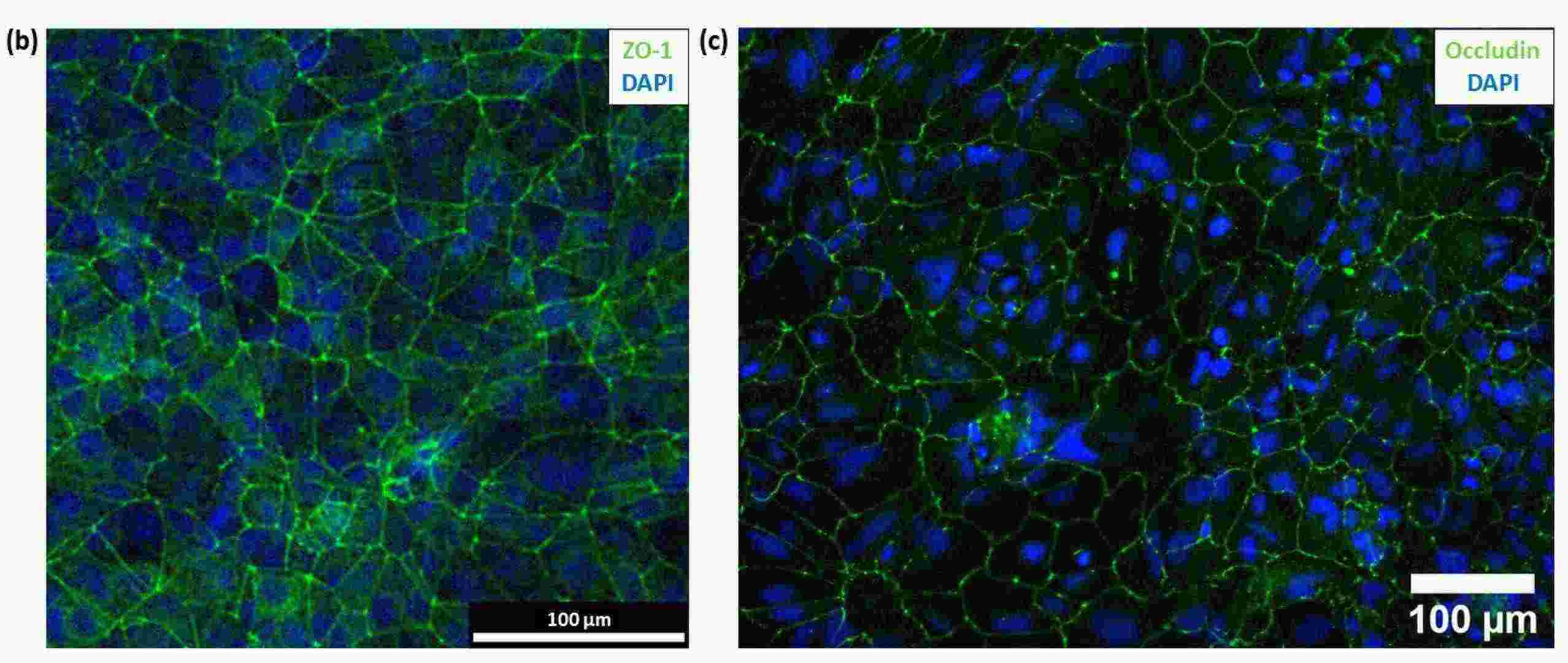 Fig. 1. (b) Immunofluorescence image of hiPSC-derived BMECs with ZO-1(green) and DAPI (blue) staining. (c) Immunofluorescence image of hiPSC-derived BMECs with occludin (green) and DAPI (blue) staining (Bull D, Schweitzer C, et al., 2022).
Fig. 1. (b) Immunofluorescence image of hiPSC-derived BMECs with ZO-1(green) and DAPI (blue) staining. (c) Immunofluorescence image of hiPSC-derived BMECs with occludin (green) and DAPI (blue) staining (Bull D, Schweitzer C, et al., 2022).
Effect of SARS-CoV-2 Infection on BMEC Integrity
SARS-CoV-2, the virus causing COVID-19, is associated with various neurological symptoms, suggesting its impact on the central nervous system (CNS) through the blood–brain barrier (BBB). The BBB is formed by tight junctions between brain microvascular endothelial cells (BMECs). There have been reports of BMEC infection with SARS-CoV-2, but its mechanism is yet to be determined.
Yamada et al. used the original SARS-CoV-2 strain to infect human iPSC-derived brain microvascular endothelial cells (iPSC-BMELCs) and assessed infection through intracellular RNA detection and viral particle localization. To evaluate the impact of SARS-CoV-2 infection on the BBB, iPSC-BMELCs were seeded into a 24-well Transwell plate to allow the cells to form a monolayer. SARS-CoV-2 infection decreased the TEER by 31% (Fig. 1A) and gradually reduced TEER values over 12 h (Fig. 1B). Immunocytochemical staining revealed that some of the infected cells were caspase-3-positive, indicating apoptosis (Fig. 1C), but they could not observe any distinct changes in TJ structure. RNA-seq analysis of SARS-CoV-2-infected iPSC-BMELCs revealed upregulation of genes related to interferon (IFN) and type I IFN signaling components. RT-qPCR showed that SARS-CoV-2 infection decreased the expression of several TJ markers, such as CLDN3 and CLDN11 (Fig. 2B), and that their protein levels were also slightly reduced (Fig. 2C). These data suggest that SARS-CoV-2 impairs BMEC barrier integrity by downregulating the expression of TJ markers.
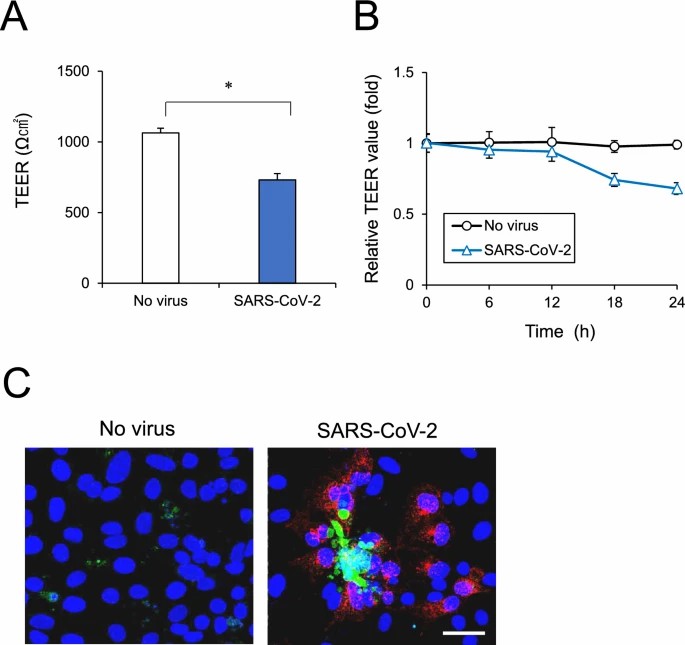 Fig. 1. BMEC monolayer barrier examined after SARS-CoV-2 infection (Yamada S, Hashita T, et al., 2024).
Fig. 1. BMEC monolayer barrier examined after SARS-CoV-2 infection (Yamada S, Hashita T, et al., 2024).
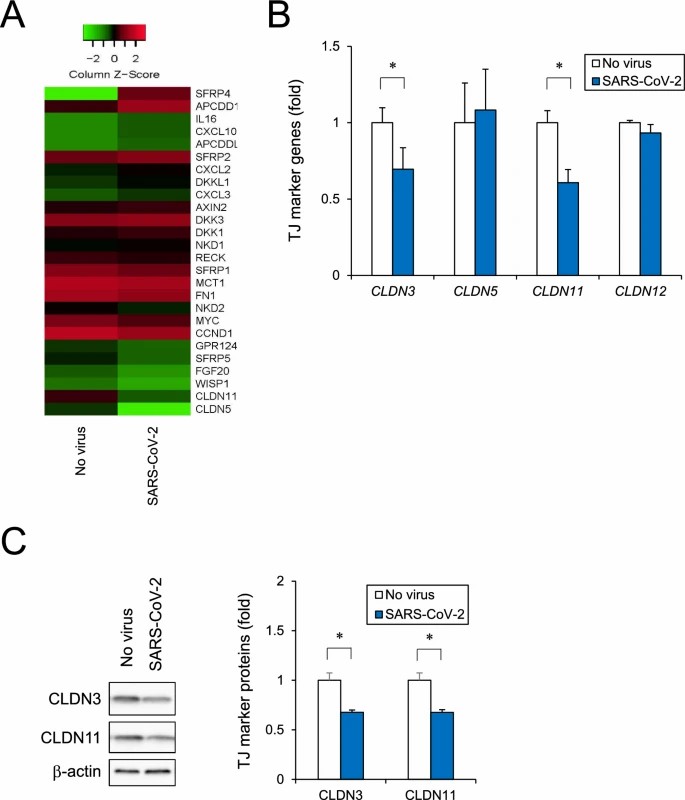 Fig. 2. Analysis of human iPSC-BMELC barrier disruption based on RNA-seq (Yamada S, Hashita T, et al., 2024).
Fig. 2. Analysis of human iPSC-BMELC barrier disruption based on RNA-seq (Yamada S, Hashita T, et al., 2024).
BMEC Maturation by Conditioned Media Results in a Barrier Tightness Similar to Co-Cultures
Current in vitro models often fail to mimic the physiological barrier properties of the BBB, especially in scalable formats. Fengler et al. aim to address this challenge by establishing a standardized and scalable multi-well plate format using human iPSC-derived brain endothelial microvessels to screen anti-inflammatory drugs for BBB penetration.
In order to generate functional BBB endothelial cells from human stem cells, they first differentiated IMR90-4 iPSCs into brain microvascular endothelial cells (BMECs) in a four-step protocol according to pervious study (Fig. 3A). After 3 days of iPSC expansion, a mixed cell culture consisting of endothelial and neuronal cell progenitors was generated by exposure to unconditioned medium for 6 days (Fig. 3B). The endothelial cells were then selectively expanded with endothelial cell medium supplemented with all-trans retinoic acid (RA) and bFGF (EC+/+). On day 8 the cultures were subcultured on collagen/fibronectin. In order to characterize the BMEC phenotype, the cells were stained for BBB marker proteins and demonstrated positive staining for typical endothelial markers (PECAM-1, VE-Cadherin), tight-junction proteins (Occludin, ZO-1, Claudin-5), nutrient transporter (Glut-1) and efflux transporters (P-gp, BCRP) (Fig. 4A). TEER measurements between day 9 and 12 revealed peak values of ~2500 Ωxcm² at day 10 (Fig. 4B). Treatment with Staurosporine (STS) abolished tight-junctions, as reflected by the reduced TEER (Fig. 3C). Overall, these results demonstrate that iPSC-derived BMECs express appropriate BBB markers and physiological barrier properties as of day 10.
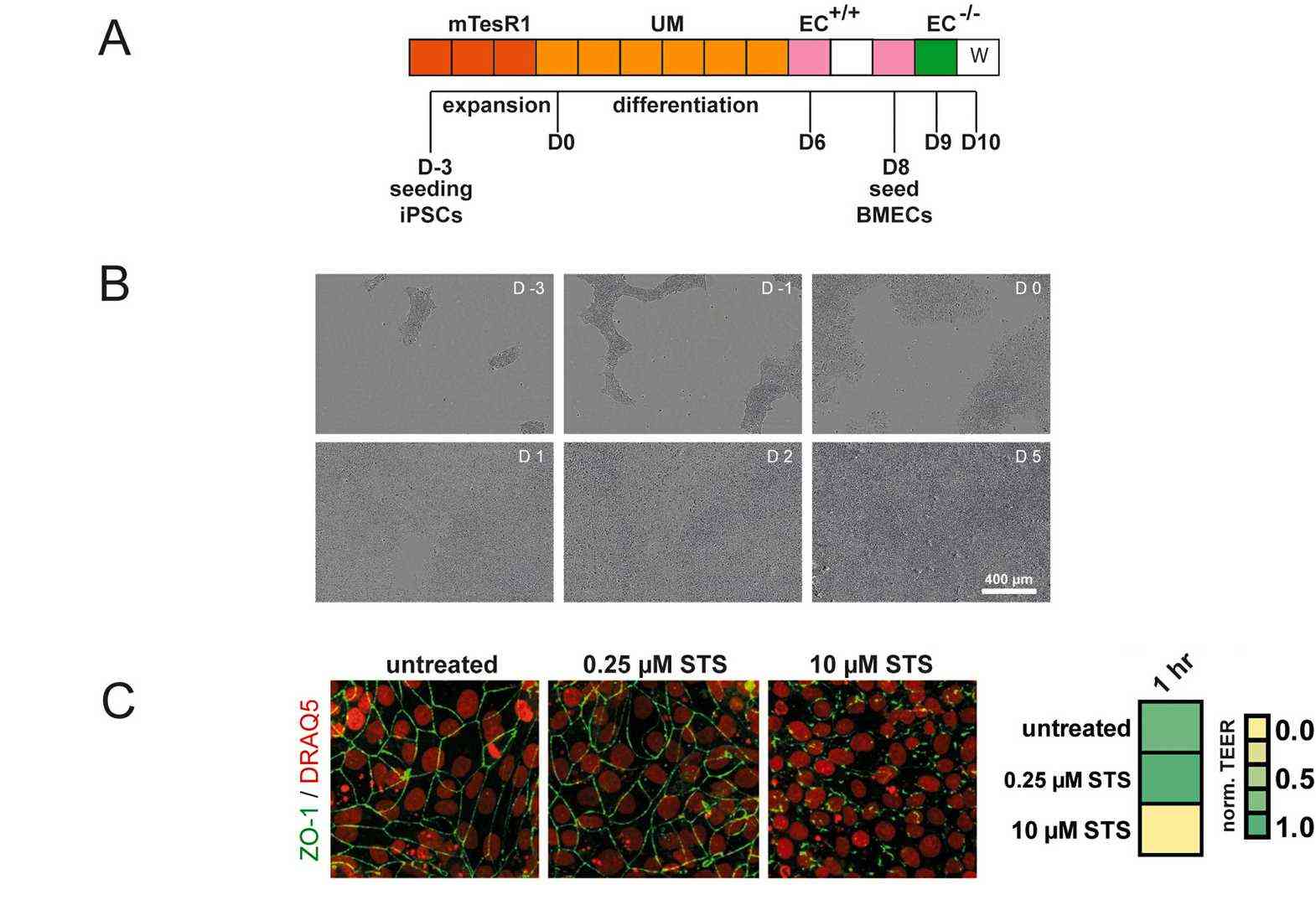 Fig. 3. (A) BMEC differentiation protocol. (B) Representative brightfield images of differentiating cells from D-3 to D5. (C) Normalized TEER measured on Transwell filters and corresponding immunocytochemistry of STS treated BMEC cultures (Fengler S, Kurkowsky B, et al., 2022).
Fig. 3. (A) BMEC differentiation protocol. (B) Representative brightfield images of differentiating cells from D-3 to D5. (C) Normalized TEER measured on Transwell filters and corresponding immunocytochemistry of STS treated BMEC cultures (Fengler S, Kurkowsky B, et al., 2022).
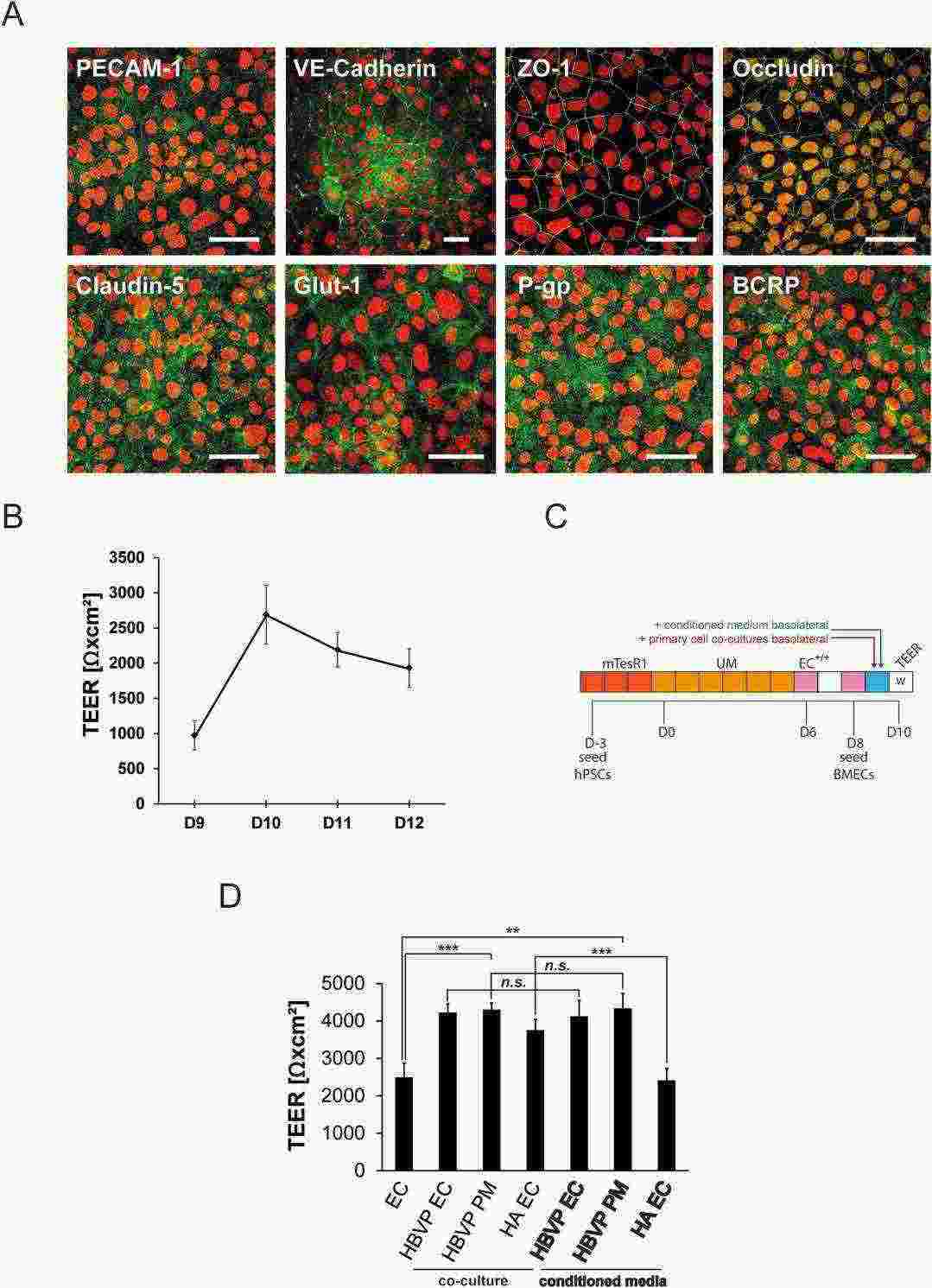 Fig. 4. Characterization of BMEC cultures and maturation with primary cell conditioned media (Fengler S, Kurkowsky B, et al., 2022).
Fig. 4. Characterization of BMEC cultures and maturation with primary cell conditioned media (Fengler S, Kurkowsky B, et al., 2022).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells