
Immortalized Mouse Retinal Cells (MU-PH1)
Cat.No.: CSC-I9277L
Species: Mus musculus
Source: Retina
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
Immortalized Mouse Retinal Cells (MU-PH1) cell line is an immortalized cell line derived from mouse retina, exhibiting characteristics of photoreceptor cells, glial cells, and stem cells. The retina serves as a vital eye component which captures light and transforms it into neural signals for brain transmission. The retina encompasses multiple cell varieties that include photoreceptor cells (like cones and rods), horizontal cells, bipolar cells plus Müller cells. Retinal development and function depend heavily on these cellular components. MU-PH1 cells maintain retinal progenitor characteristics and express several markers including photoreceptor markers such as rhodopsin and chromoproteins. Neural stem cell markers feature proteins such as nestin and β-III tubulin in addition to glutamine synthetase and glial cell markers like S-100 protein and glutamine synthetase.
The expression of retina-specific markers in MU-PH1 cells makes them a common choice for research on retinal degenerative diseases. For instance, researchers employ MU-PH1 cells in retinitis pigmentosa (RPE) models to investigate how miR-6937-5p affects photoreceptor health and retinal functionality. The neural stem cell properties of these cells allow researchers to investigate retinal neuroregeneration possibilities. Induced differentiation enables these cells to produce photoreceptors and additional retinal neurons which could serve as a cellular resource for retinal restoration.
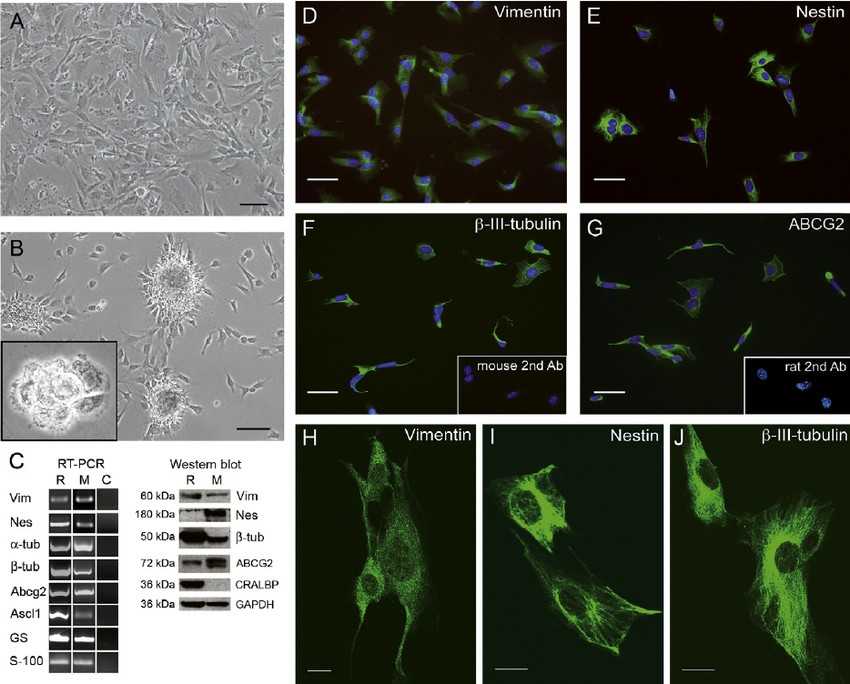 Fig. 1. Characterization of the MU-PH1 cell line (Gomez-Vicente V, Flores A, et al., 2013).
Fig. 1. Characterization of the MU-PH1 cell line (Gomez-Vicente V, Flores A, et al., 2013).
Inhibition of MicroRNA 6937 Delays Photoreceptor and Vision Loss in a Mouse Model of Retinitis Pigmentosa
Inherited retinal dystrophies (IRDs), such as retinitis pigmentosa (RP), cause progressive vision loss due to genetic mutations. Despite advances in gene therapy, effective treatments for all IRDs remain elusive. MicroRNAs (miRNAs), known for regulating gene expression, play critical roles in various physiological and pathological processes including retinal diseases.
Anasagasti et al. evaluated whether modulating miRNAs can protect photoreceptor cells in the rd10 mouse model of RP, offering insights into novel therapeutic strategies for IRDs. The results obtained indicate that inhibition of the miR-6937-5p slows down the visual deterioration of rd10 mice, reflected by an increased electroretinogram (ERG) wave response under scotopic conditions and significant preservation of the outer nuclear layer thickness. Finally, to test the effect on cellular viability upon increased expression of miR-6937-5p, they used a photoreceptor-like cell line, MU-PH1, generously provided by Professor Nicolas Cuenca from U. of Alicante, Spain. MU-PH1 cells were transfected with miR-6937-5p mimic using the miRIDIAN system. After 48 h of incubation with miRIDIAN miR-6937-5p Mimic Transfection, a 174-fold increase in the expression of miR-6937-5p was achieved in 29.9% of MU-PH1 cells, compared with cells treated with miRNA mimic negative control. This increased expression of miR-6937-5p was correlated with increased cytotoxicity of MU-PH1 cells (Fig. 1).
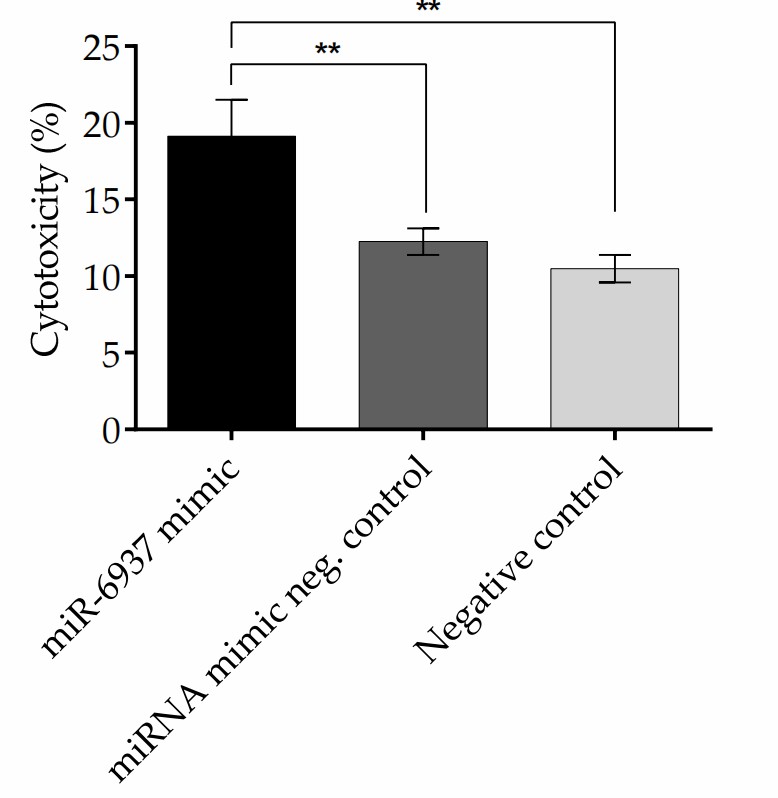 Fig. 1. Cytotoxicity of MU-PH1 cell line treated with miR-6937-5p mimics (Anasagasti A, Lara-López A, et al., 2020).
Fig. 1. Cytotoxicity of MU-PH1 cell line treated with miR-6937-5p mimics (Anasagasti A, Lara-López A, et al., 2020).
TLR2 Activation Increases Neurosphere Formation in MU-PH1 Cell Line
It is well known that TLRs recognize molecular signatures of microbial pathogens and induce the activation of the innate immune responses, as well as the subsequent adaptive immune responses. TLR2, which forms TLR2/TLR6 heterodimers, is involved in the signaling induced by C. albicans. Consequently, Gómez-Vicente et al. investigated TLR2 and TLR6 gene expression upregulation in MU-PH1 cells exposed to C. albicans. Both heat-killed (Fig. 2C) and viable (Fig. 2E) C. albicans ATCC 26555 increased TLR2 and TLR6 expression, as shown by RT-PCR (Fig. 2D, F). TNF-α, a cytokine produced upon microbial recognition by TLRs, was tested for production in MU-PH1 cells exposed to TLR ligands and inactivated C. albicans for up to 6 days. No morphological changes or TNF-α secretion were detected. In retinal progenitor cells, TLR4 limits proliferation and neuronal differentiation in vitro. To explore TLR2's role, we examined the effect of heat-killed C. albicans ATCC 26555 and Pam(3)CysSK(4) on MU-PH1 cells' neurosphere formation. Yeast cells significantly increased neurosphere numbers by 83.9 ± 22.9%, while Pam(3)CysSK(4) raised them by 86.1 ± 19.7%, both statistically significant with p < 0.05 (Fig. 3).
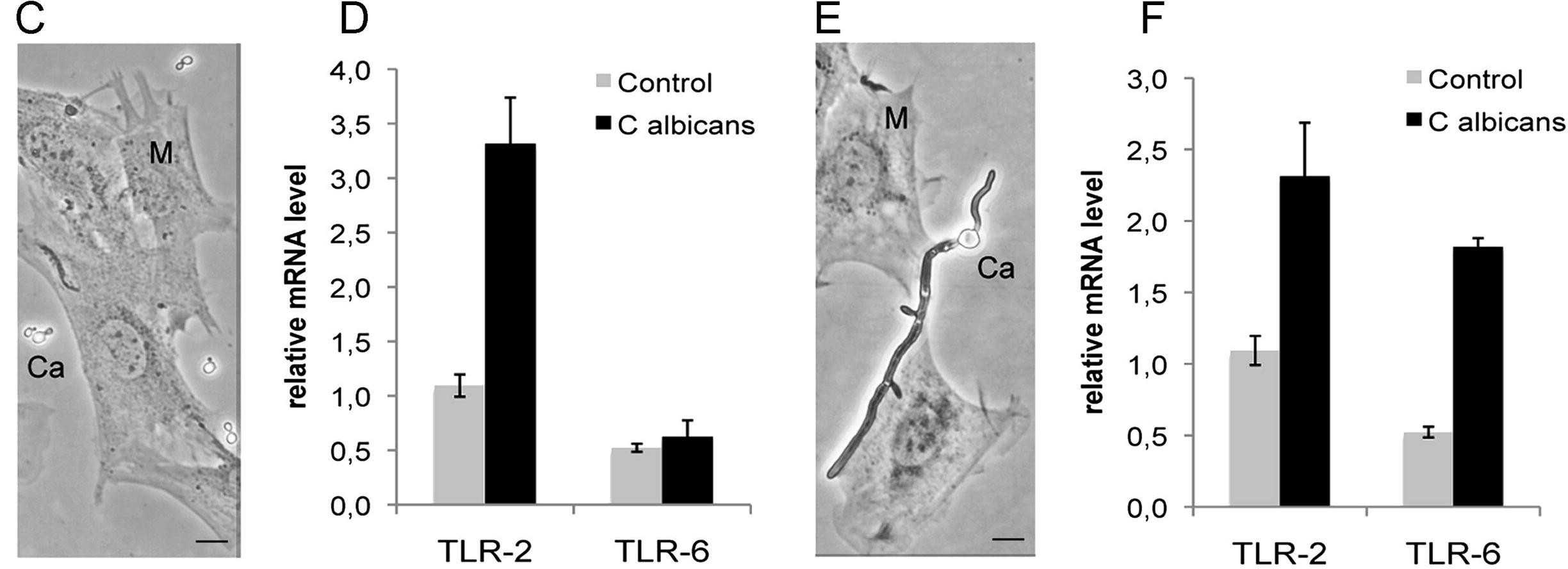 Fig. 2. TLRs are expressed in MU-PH1 cell lines (Gomez-Vicente V, Flores A, et al., 2013).
Fig. 2. TLRs are expressed in MU-PH1 cell lines (Gomez-Vicente V, Flores A, et al., 2013).
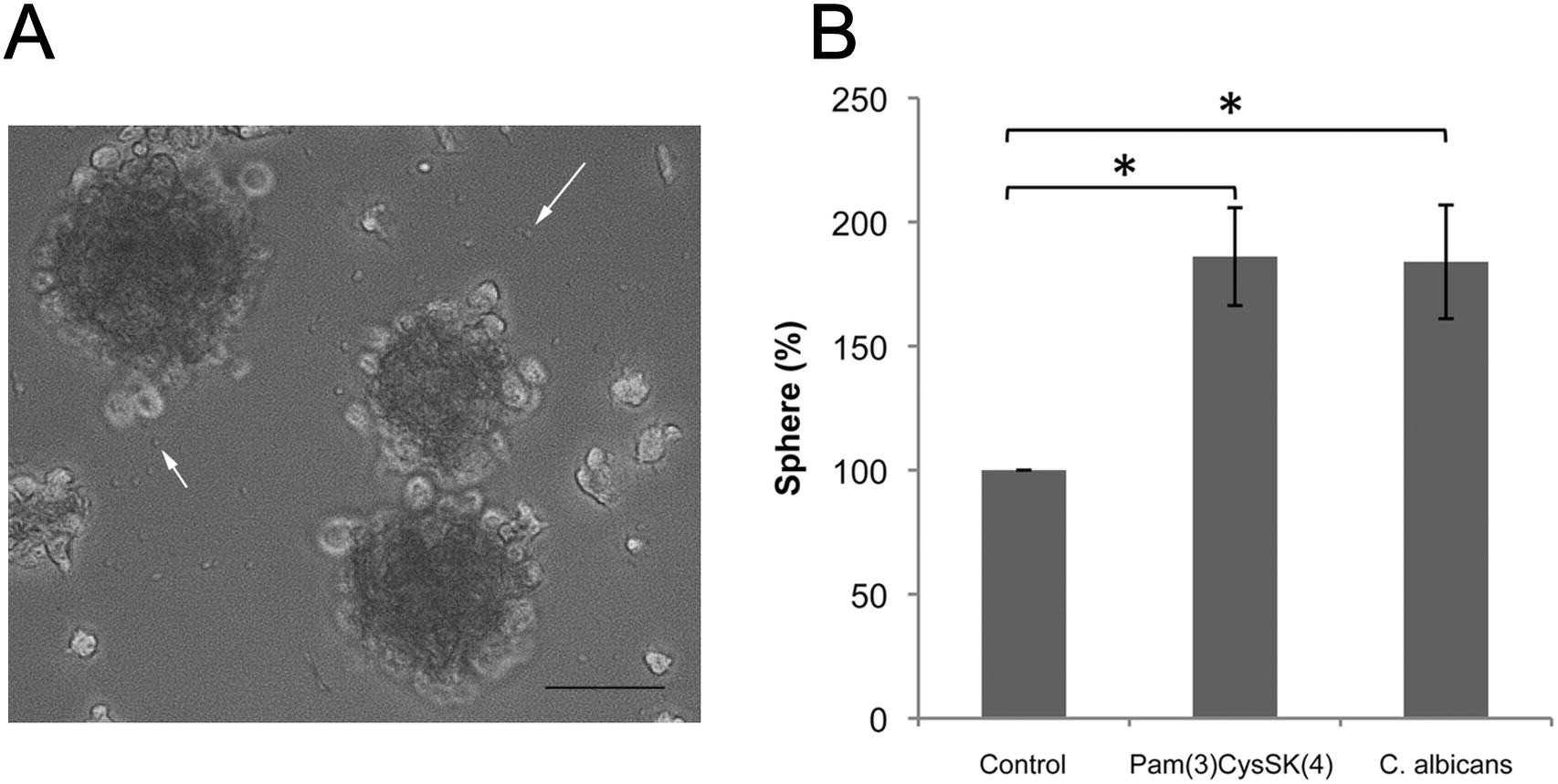 Fig. 3. TLR2 ligands increase neurosphere formation (Gomez-Vicente V, Flores A, et al., 2013).
Fig. 3. TLR2 ligands increase neurosphere formation (Gomez-Vicente V, Flores A, et al., 2013).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

