
Immortalized Mouse Distal Caput Epididymal Epithelial Cell Line (DC2)
Cat.No.: CSC-I9202L
Species: Mus musculus
Source: caput epididymis
Morphology: polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT004 HT® Lenti-SV40 (tsA58 temperature sensitive mutant) Lentivirus Immortalization Kit
2) RT-PCR was used to assess the T-antigen transgene expression in the immortalized cell line.
The Immortalized Mouse Distal Caput Epididymal Epithelial Cell Line (DC2) comes from transgenic mice's distal caput epididymis region with temperature-sensitive large T antigen of Simian Virus 40 (SV40). The epididymis, a highly convoluted duct located between the testis and the vas deferens, is structurally divided into four main parts: the initial segment, caput, corpus, and cauda. The DC2 cell line develops from the distal caput section of the epididymis which serves as a key site for sperm maturation.
DC2 cells release various proteins and small non-coding RNAs (sncRNAs), which enter sperm through vesicles to affect sperm maturation and functional capabilities. For example, the miR-141 miRNA produced by DC2 cells can control sperm epigenetic conditions which influence offspring health. Moreover, DC2 cells have a strong reaction to environmental stressors such as cortisol and they pass stress signals to sperm through the release of vesicles. Research demonstrates that chronic stress in male parents before conception alters miRNA expression in DC2 cell vesicles which passes to offspring sperm and influences their neurodevelopment. Additionally, the DC2 cells host multiple genes and proteins that control cell cycle functions including ZBTB17 and TP53 which serve essential functions in the
Epididymal Glucocorticoid Receptors Promote Intergenerational Transmission of Paternal Stress
Paternal preconception exposures, including stress and diet, can shape offspring health outcomes. Mechanistic studies suggest that small noncoding RNAs in sperm, such as microRNAs (miRs), carry paternal environmental information. However, the cellular mechanisms of how these signals are relayed to sperm and persist remain unknown.
To identify the molecular and epigenetic marks involved in the persistence of sperm miR alterations by stress, Chan et al. exposed male mice to chronic stress and collected epididymal sperm 1 or 12 weeks post-stress (Fig. 1a, top) to compare acute and enduring effects. Small RNA sequencing revealed two distinct populations of differentially expressed sperm miRs with no shared stress-altered miRs between them (Fig. 1b), suggesting a unique mechanism induces enduring changes in sperm miR populations following the acute stress response. To investigate how epigenetic changes in epididymal extracellular vesicles (EVs) are maintained after paternal stress, they treated DC2 mouse caput epididymal epithelial cells with corticosterone in vitro. This allowed them to isolate EVs from a specific cell population and control corticosterone administration. They tested three corticosterone concentrations (low, medium, and high) at three time points to examine acute, intermediate, and enduring effects (Fig. 1a, bottom). Using rank-rank hypergeometric overlap (RRHO) analyses, they compared in vivo stress-altered miRs with in vitro corticosterone-altered miRs. The overlap increased dramatically at 8 days compared to 1-day post-treatment (Fig. 1c). The 8-day time point with stress-relevant corticosterone concentration most closely matched in vivo enduring paternal stress effects (Fig. 1d), with 31.4% overlapping miRs (116/369). This suggests enduring effects in the in vitro model, though sperm miR complexity reflects additional interactions along the reproductive tract.
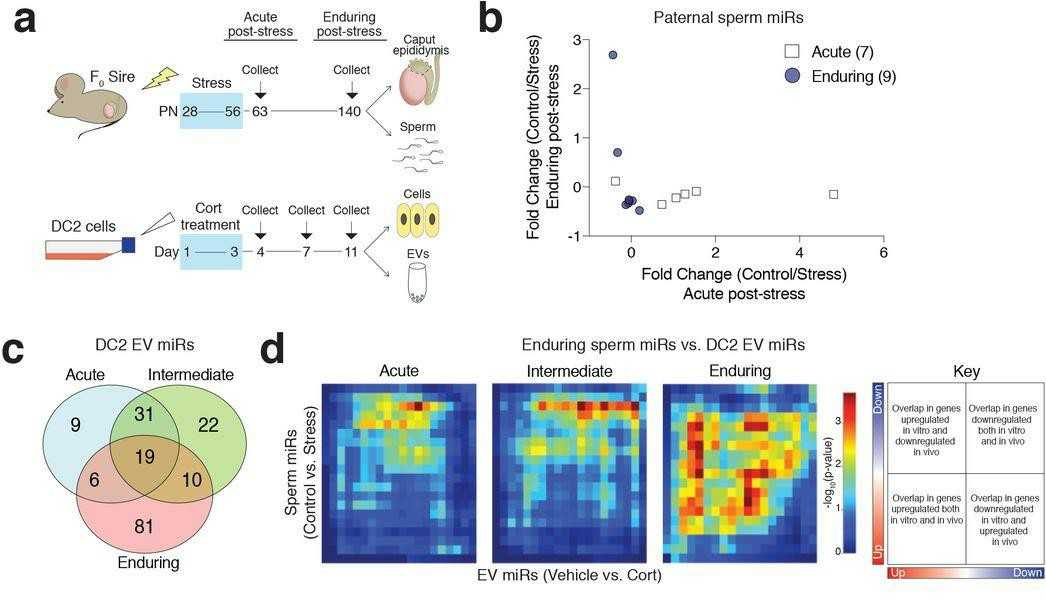 Fig. 1. Glucocorticoid-treated DC2 mouse caput epididymal epithelial cell EVs in vitro mimic paternal stress programming of enduring sperm miRs in vivo (Chan JC, Nugen BM, et al., 2018).
Fig. 1. Glucocorticoid-treated DC2 mouse caput epididymal epithelial cell EVs in vitro mimic paternal stress programming of enduring sperm miRs in vivo (Chan JC, Nugen BM, et al., 2018).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

