Immortalized Mouse Dendritic Cells (DC2.4 )
Cat.No.: CSC-I2319Z
Species: Mouse
Culture Properties: Adherent and suspension
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Free from contaminations (bacteria incl. mycoplasma, fungi, HIV, HAV, HBV, HCV, Parvo-B19) and cross-contaminations.
Dendritic cells (DCs) are a heterogeneous lineage of innate immune cells with a unique and essential role in the initiation and coordination of the adaptive immune response. Upon encountering pathogens, DCs become activated and undergo a series of functional modifications that include induction of cytokine and chemokine production, regulation of surface marker expression and increase of antigen presentation efficiency.
One of the biggest challenges in the study of DCs is the difficulty to obtain sufficient quantities of viable and non-activated cells for experimentation. Indeed, DCs are rather scarce in vivo and they are very sensitive to prolonged culturing in vitro, prone to spontaneous activation and cell death. For these reasons, in the past years, considerable effort has been dedicated to simplifying the access to sufficient quantities of DCs in vivo and in vitro. The generation of immortalized DC lines is a valuable alternative that overcome some of the technical challenges associated with these methods, like the sensitivity and the functional instability of DCs generated in vitro, as well as the need for long differentiation protocols and the potentially tedious cell purification steps.
The DC2.4 cell line, derived from C57BL/6 mice, is an immortalized murine DC line generated by retrovirus transduction of oncogenes myc and raf. DC2.4 exhibits typical characteristics of dendritic cells, including cell morphology, expression of dendritic cell-specific markers, and the ability to phagocytose and present exogenous antigens on both MHC class I and class II molecules. This makes them very useful for investigating the mechanisms and stimuli required for DC maturation and their subsequent immune functions.
Utilizing Murine Dendritic Cell Line DC2.4 to Evaluate the Immunogenicity of Subunit Vaccines In Vitro
Subunit vaccines hold great potential for controlling infectious diseases due to their superior safety, specific immunogenicity, simplified manufacturing processes, and well-defined chemical compositions. One of the most important targets of vaccines is a subset of lymphocytes originating from the thymus, known as T cells, which are able to elicit antigen-specific immune response. Dendritic cells (DCs) are essential for the activation of T cells and the induction of adaptive immunity, making them key for the in vitro evaluation of vaccine efficacy. Upon internalization by DCs, vaccine-bearing antigens are processed, and suitable fragments are presented to T cells by major histocompatibility complex (MHC) molecules. In addition, DCs can secrete various cytokines to crosstalk with T cells to coordinate subsequent immune responses. Here, we generated an in vitro model using the immortalized murine dendritic cell line, DC2.4, to recapitulate the process of antigen uptake and DC maturation, measured as the elevation of CD40, MHC-II, CD80 and CD86 on the cell surface. The levels of key DC cytokines, tumor necrosis alpha (TNF-α) and interleukin-10 (IL-10) were measured to better define DC activation. This information served as a cost-effective and rapid alternative for assessing the antigen presentation efficacy of various vaccine formulations.
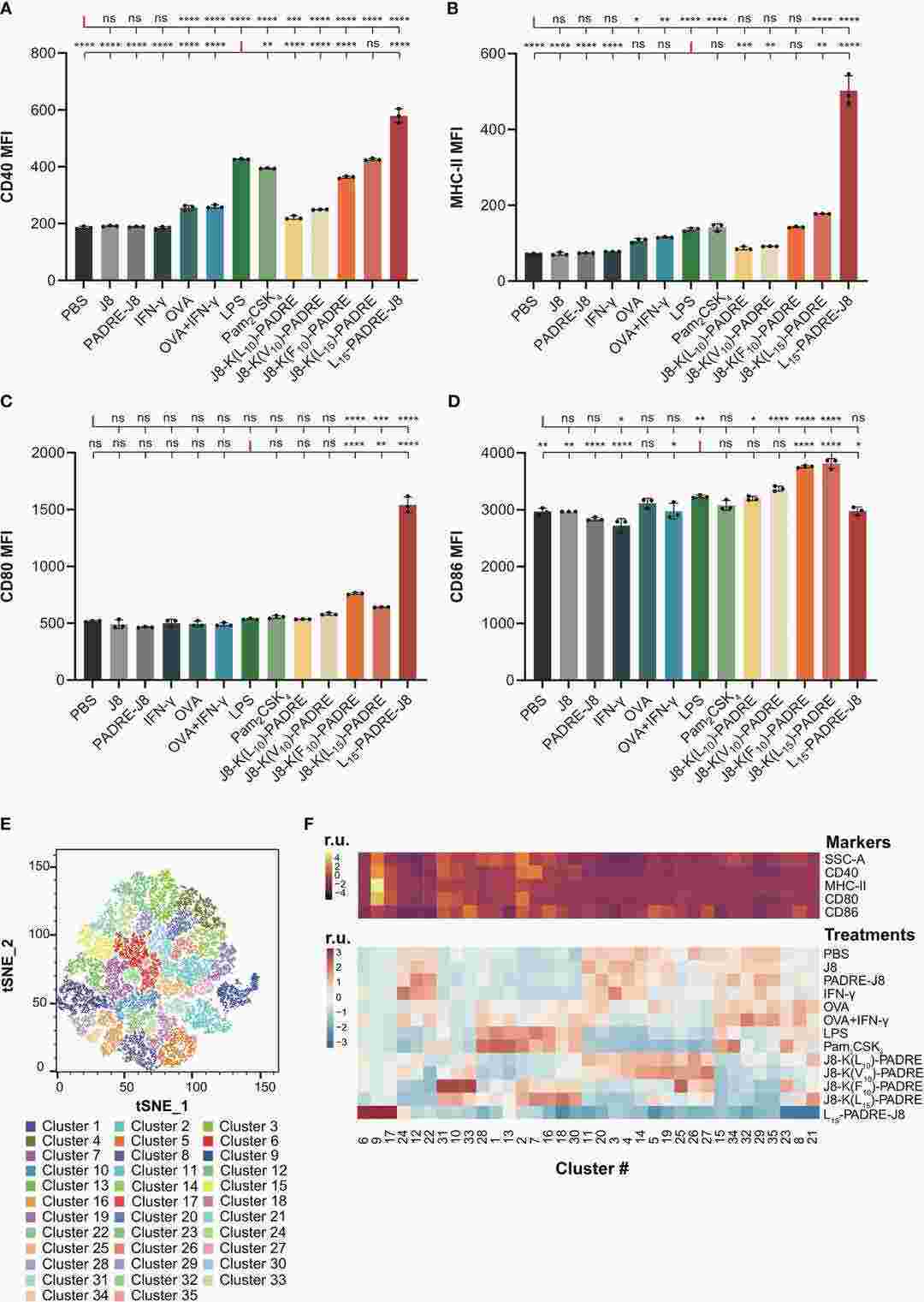 Fig. 1. Maturation of DC2.4 cells by peptide vaccines (Lu, Lantian, et al. 2024).
Fig. 1. Maturation of DC2.4 cells by peptide vaccines (Lu, Lantian, et al. 2024).
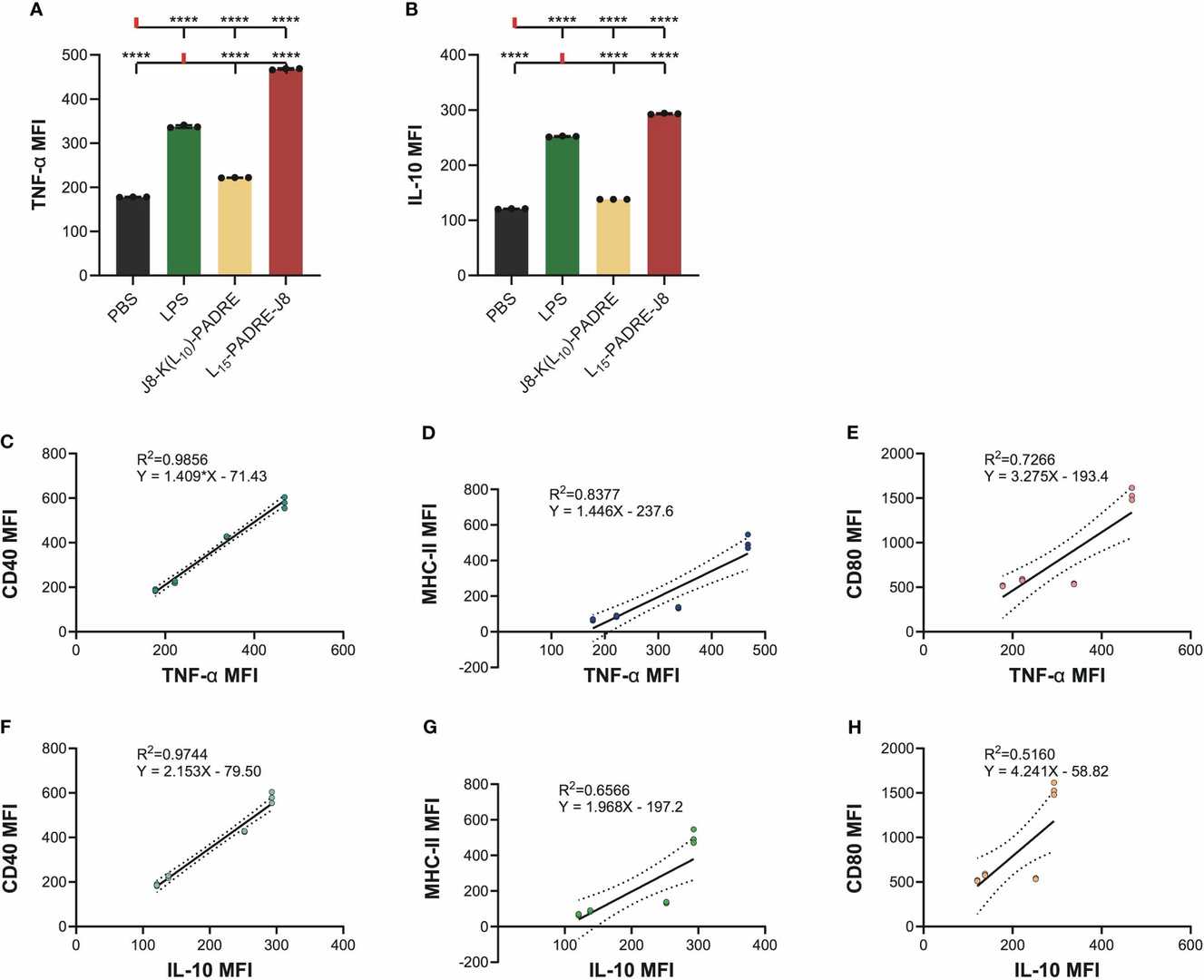 Fig. 2. Intracellular cytokine staining of cells treated with PBS, LPS, J8-K(L10)-PADRE, and L15-PADRE-J8 (Lu, Lantian, et al. 2024).
Fig. 2. Intracellular cytokine staining of cells treated with PBS, LPS, J8-K(L10)-PADRE, and L15-PADRE-J8 (Lu, Lantian, et al. 2024).
Flagella Protein Promote Dendritic Cell Activation and Functional Maturation Through the TLR5 Receptor
Flagellin protein is the main component of the flagellar structure of flagellated bacteria. The highly conserved TLR recognition site on the flagellar structure can specifically bind to Toll-like receptor 5 (TLR5) on dendritic cells in the lamina propria, thereby playing an important role in the immune response against pathogenic bacteria in the intestinal mucosa and leading to chronic inflammatory reactions in the intestinal mucosa. The objective is to explore whether the flagellin-TLR5 complex signal can enhance the antigen presentation ability of myeloid DCs through the TRIF-ERK1/2 pathway, and the correlation between this pathway and intestinal mucosal inflammation response.
Mouse bone marrow-derived DC line DC2.4 was divided into four groups: control group (BC) was DC2.4 cells cultured normally; flagellin single signal stimulation group (DC2.4+CBLB502) was DC2.4 cells stimulated with flagellin derivative CBLB502 during culture; TLR5-flagellin complex signal stimulation group (ov-TLR5-DC2.4+CBLB502) was flagellin derivative CBLB502 stimulated ov-TLR5-DC2.4 cells with TLR5 gene overexpression; TRIF signal interference group (ov-TLR5-DC2.4+CBLB502+Pepinh-TRIFTFA) was ov-TLR5-DC2.4 cells with TLR5 gene overexpression stimulated with flagellin derivative CBLB502 and intervened with TRIF-specific inhibitor Pepinh-TRIFTFA.
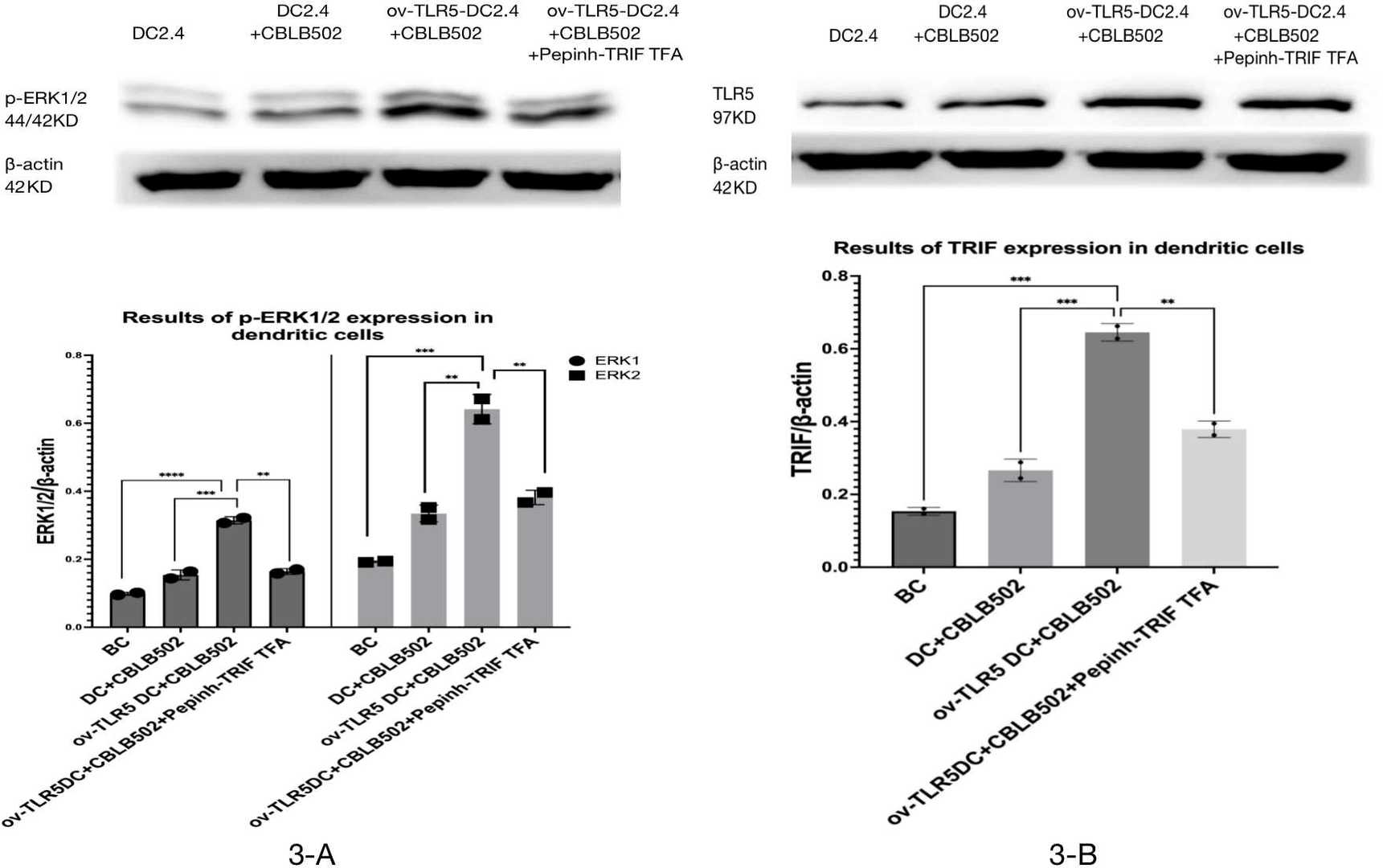 Fig. 3. Expression of TRIF and p-ERK1/2 proteins in each group of dendritic cells (Zhuang, Zhaomeng, et al. 2024).
Fig. 3. Expression of TRIF and p-ERK1/2 proteins in each group of dendritic cells (Zhuang, Zhaomeng, et al. 2024).
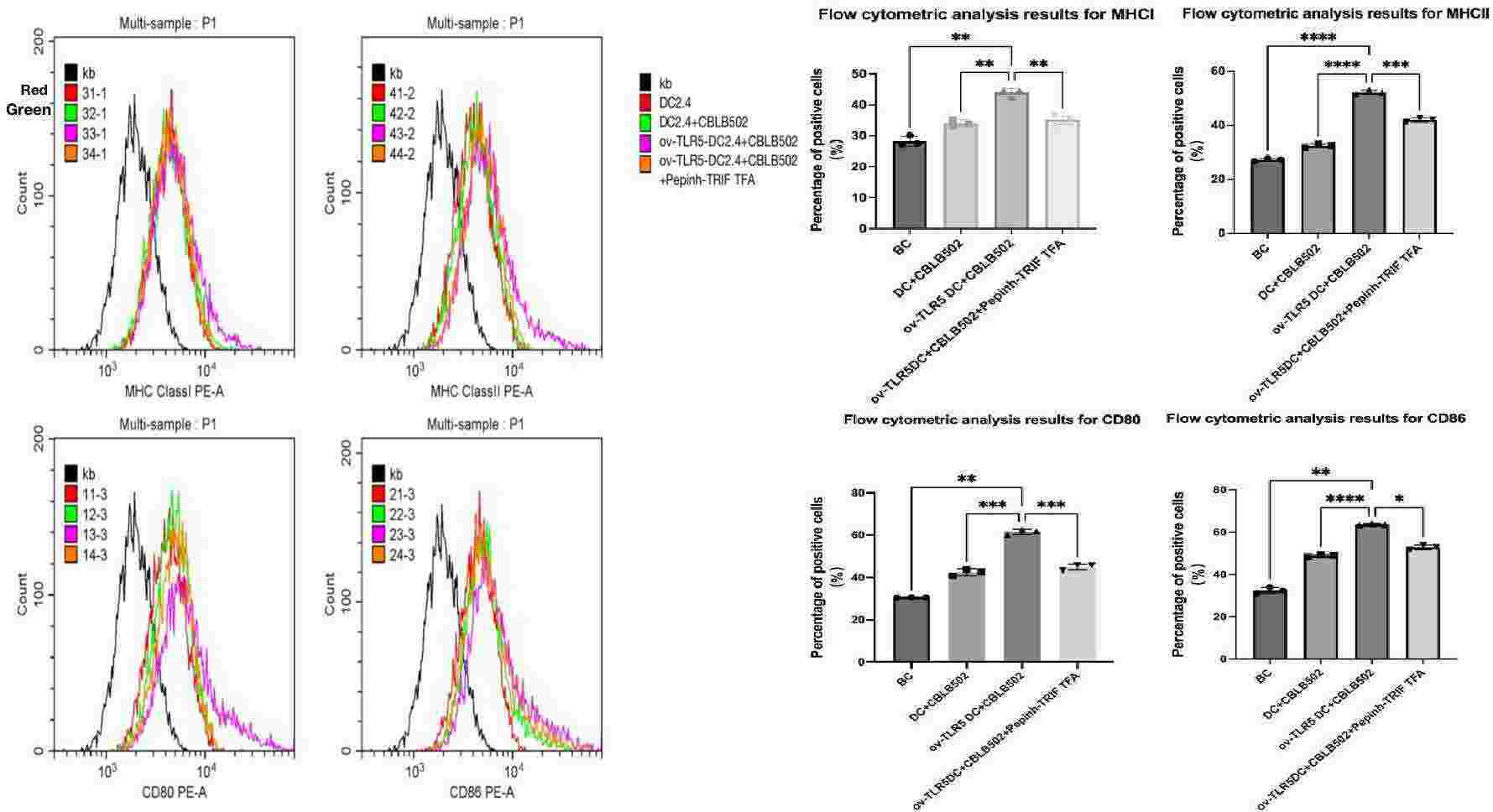 Fig. 4. Expression of MHCI, MHCII, CD80, and CD86 molecules on the surface of dendritic cells in each group (Zhuang, Zhaomeng, et al. 2024).
Fig. 4. Expression of MHCI, MHCII, CD80, and CD86 molecules on the surface of dendritic cells in each group (Zhuang, Zhaomeng, et al. 2024).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells