
Immortalized Mouse Cortical Collecting Duct Cell Line (mCCD(cl1))
Cat.No.: CSC-I9092L
Species: Mus musculus
Source: Kidney
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT003 HT® Lenti-SV40T Immortalization Kit
2) RT-PCR was used to detect expression of MR, GR, and 11β-HSD2;
3) sodium transport response assayed through short-circuit current (electrophysiologic studies) and binding assays.
The mCCD(cl1) cell line, an immortalized mouse cortical collecting duct line, was isolated from mouse kidney and spontaneously immortalized. It highly expresses mineralocorticoid (MR) and glucocorticoid (GR) receptors, and 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) and responds to aldosterone and other hormones, faithfully recapitulating physiological sodium transport in vivo. Furthermore, mCCD(cl1) cells also express the epithelial sodium channel (ENaC) and Na⁺/K⁺-ATPase, both of which are pivotal for sodium handling. For these reasons, the cell line has been employed in research to understand renal physiology, ion transport, hormone signaling and mechanisms of kidney-related diseases, among others, aldosterone effects on sodium transport, receptor expression and function, and kidney disease models.
Downregulation of Hemin-Induced Nrf2 Pathway Activation Decreases Hepcidin Synthesis
The release of hemoglobin during hemolysis can lead to deposition in renal tubules, causing oxidative stress and AKI. It has been reported that urinary hepcidin levels are elevated in patients with AKI, which indicates the protective role of kidney-derived hepcidin, but the regulatory mechanism is not yet known. The Nrf2 pathway is crucial for cellular stress defense and regulates hepcidin synthesis in the liver. Iron exposure activates Nrf2 in proximal tubule cells, and hemin induces hepcidin synthesis. Diepeveen et al. explored whether Nrf2 mediates hemin-induced hepcidin expression in mCCDcl1 cells and how Nrf2 modulation affects this synthesis, using the inhibitor trigonelline.
Treatment with hemin increased HO-1 mRNA expression as an indicator of heme catabolism and oxidative stress, which was attenuated by co-incubation with trigonelline (Fig. 1A). Nrf2 activation was shown by an increase in mRNA of target genes TXNRD1, GCLM and NQO1, and increase in NQO1 protein (Fig. 1B, C). Trigonelline caused a significant decrease in KEAP1 and GCLM mRNA, and NQO1 protein expression (Fig. 1B, C). Nuclear Nrf2 protein levels increased after incubation with hemin while the total cellular Nrf2 levels did not change (Fig. 1D). Co-incubation with trigonelline significantly reduced the Nrf2 translocation, however, this effect was also seen in control cells, so the data suggests that hemin activates the Nrf2 pathway in mCCDcl1 cells, which can be inhibited by trigonelline. Hemin significantly increased hepcidin mRNA expression, as well as luciferase activity, both of which were decreased after treatment with trigonelline. The data presented above suggest that hepcidin synthesis in these cells is dependent on Nrf2 activation and occurs after hemin-induced injury.
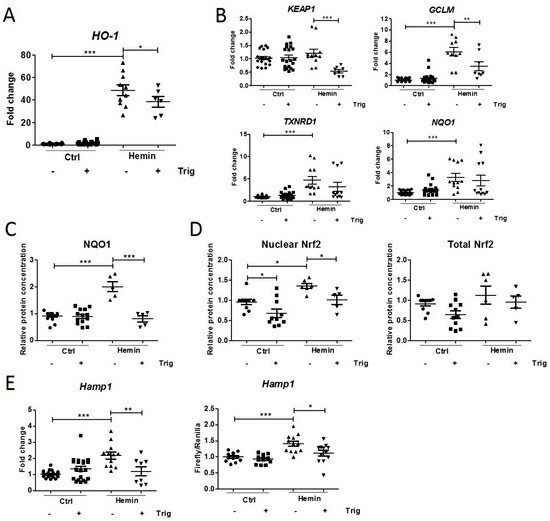 Fig. 1. Hemin-induced Nrf2 pathway activation and subsequent downregulation using trigonelline in mCCDcl1 cells (Diepeveen LE, Stegemann G, et al., 2022).
Fig. 1. Hemin-induced Nrf2 pathway activation and subsequent downregulation using trigonelline in mCCDcl1 cells (Diepeveen LE, Stegemann G, et al., 2022).
Aldosterone Increases Expression of Claudin-8 in Cultured Mouse CD Principal Cells
The aldosterone-sensitive distal nephron, a major site of transcellular Na+ reabsorption, tightly couples its activity to the tight-junction protein claudin-8, but the molecular mechanism is unknown. To test the hypothesis that aldosterone signaling through the mineralocorticoid receptor and the kinases GSK-3, Lyn and Abl up-regulates claudin-8 to strengthen the paracellular Na+ barrier.
Sassi et al. investigated how aldosterone regulates the expression of tight-junction core proteins in collecting-duct principal cells (mCCDcl1). Aldosterone augmented transepithelial current without affecting transepithelial resistance (Fig. 2A). Blockade of ENaC activity with 10⁻⁵ M benzamil only slightly increased resistance in control and aldosterone-treated monolayers, indicating that ENaC activity only modestly contributes to resistance. Of the three major claudins expressed in collecting ducts (claudin-4, -7 and -8), only claudin-8 was regulated: aldosterone time-dependently increased claudin-8 protein and mRNA, while claudin-4, claudin-7 and the tight-junction scaffold protein ZO-1 were unchanged (Fig. 2B, D). Immunofluorescence confirmed that the increase of claudin-8 occurred along the lateral membrane (Fig. 2C). The aldosterone-induced up-regulation of the canonical target Sgk-1 served as a positive control (Fig. 2B, C).
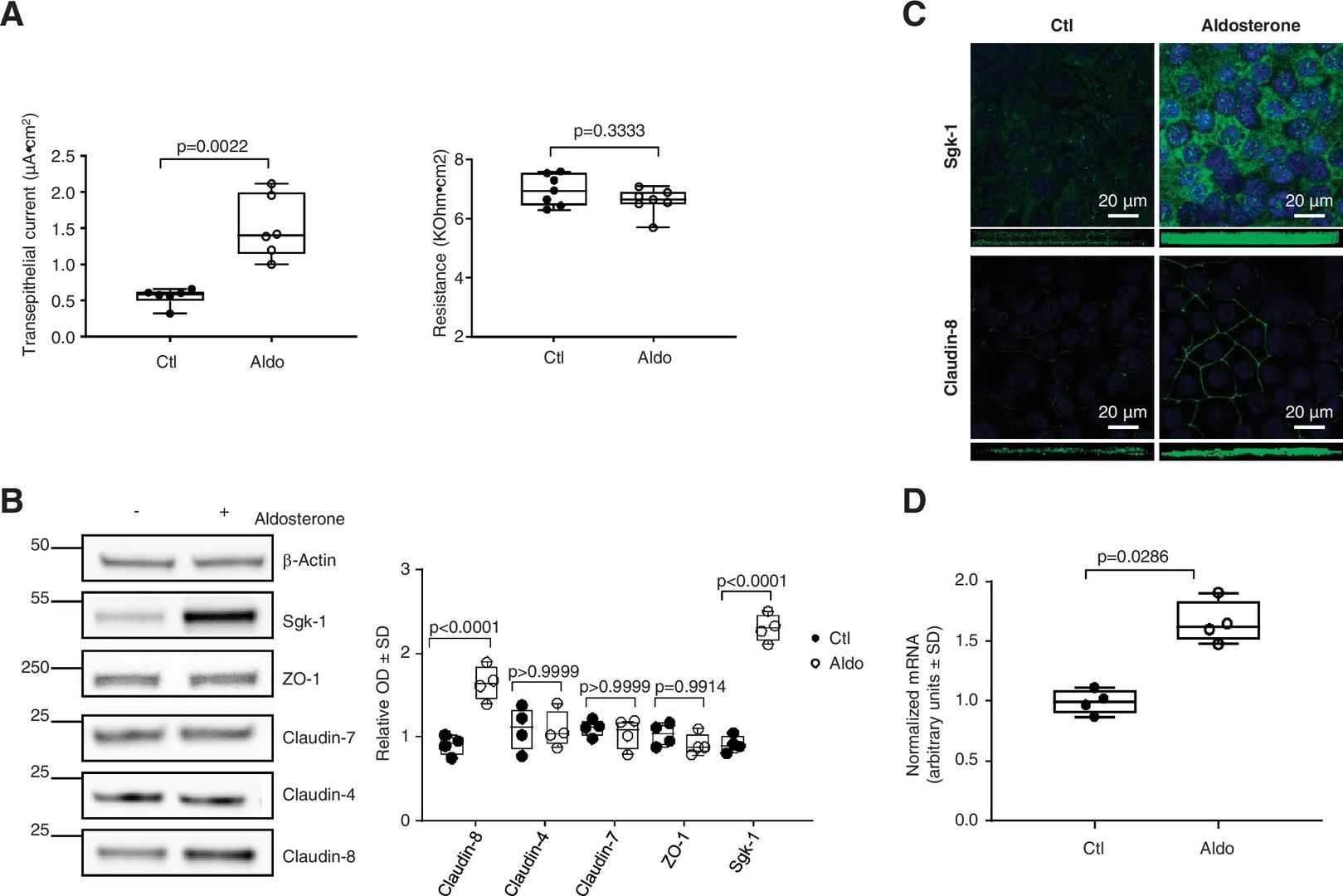 Fig. 2. Aldosterone (Aldo) modulates the expression of claudin-8 in cultured mouse collecting duct principal cells. mCCDcl1 cells were grown to confluence on filters and treated or not treated with 10−6 M aldosterone for 24 h (Sassi A, Wang Y B, et al., 2021).
Fig. 2. Aldosterone (Aldo) modulates the expression of claudin-8 in cultured mouse collecting duct principal cells. mCCDcl1 cells were grown to confluence on filters and treated or not treated with 10−6 M aldosterone for 24 h (Sassi A, Wang Y B, et al., 2021).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells
