O9-1 Mouse Cranial Neural Crest Cell Line
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can primary cells be kept at -20 °C.
O9-1 cell line is derived from cranial neural crest cells of mouse embryos, initially isolated using the Wnt1-Cre/R26R-GFP marking system from E8.5 mouse embryos. The O9-1 cell line maintains an undifferentiated stem cell phenotype in vitro and shows morphological changes shifting between epithelial-like to cuboidal or flat shapes. With the addition of differentiation-inducing factors, significant morphological changes occur. For instance, under osteogenic conditions, the cells form compact clusters and gradually develop bone-like nodular structures, expressing osteogenic markers such as alkaline phosphatase (ALP) and osteocalcin (OCN). When cultivated under chondrogenic conditions O9-1 cells express cartilage markers Sox9 and Agc1 to develop hyaline cartilage structures. When induced towards smooth muscle differentiation, O9-1 cells can express smooth muscle actin (αSMA) and other smooth muscle-specific markers. Additionally, O9-1 cells demonstrate the ability to stay undifferentiated for long durations in vitro while expressing characteristics of neural crest stem cells through markers CD44, Sca-1 and Bmi1.
Researchers commonly use the O9-1 cell line to investigate craniofacial structure formation mechanisms. For example, they inject O9-1 cells into chick embryos or mouse cranial tissues to monitor their differentiation behavior within craniofacial tissues. Using gene editing technologies, such as CRISPR-Cas9 and siRNA, researchers can explore the signaling pathways regulating neural crest cell differentiation, such as the effectors Yap and Taz in the Hippo signaling pathway. Moreover, O9-1 cells are also used to construct vascularized dentin-pulp complex tissues, providing new strategies for tooth regeneration.
O9-1 Cells Contribute to Tooth Germ Formation in vitro
Damage or loss of teeth profoundly affects life quality, and restoring them conventionally relies on non-biological materials. NCCs, integral in embryonic development, have been explored for their potential in tissue regeneration. Zhang's team utilized O9-1 mouse cranial neural crest cell line and iPSC-derived neural crest-like cells (iNCLCs) to study their differentiation into odontoblasts and formation of dentin-pulp complexes.
To explore the role of various mesenchymal cell populations in forming bioengineered tooth germ, they mixed O9-1 cells, hDPSCs, or NIH3T3 with E14.5 dental mesenchymal cells at 25, 50, and 100% ratios with E14.5 dental epithelium (Fig. 1A). Tooth germ formation was observed in mixtures with 25% NIH3T3 and 25% or 50% O9-1 cells. No GFP-labeled NIH3T3 cells were found in the germ (Fig. 1B), indicating they don't contribute to formation. No tooth germ formed with hDPSCs at any ratio. To further examine O9-1 cells' role, they used red fluorescence-labeled dental epithelium. E14.5 dental germs were dissected, and mesenchyme was mixed with O9-1 cells at a 1:1 ratio (Fig. 2A and B). In organ cultures, red fluorescent epithelium and green GFP-labeled O9-1 cells were observed in the developing tooth germ (Fig. 2C and D). O9-1 cells integrated with the germ and showed characteristics of odontoblasts, confirmed by DMP-1 staining (Fig. 2D). This indicates that O9-1 cells, a mouse cranial neural crest cell line, can differentiate into odontoblasts and contribute to tooth germ formation.
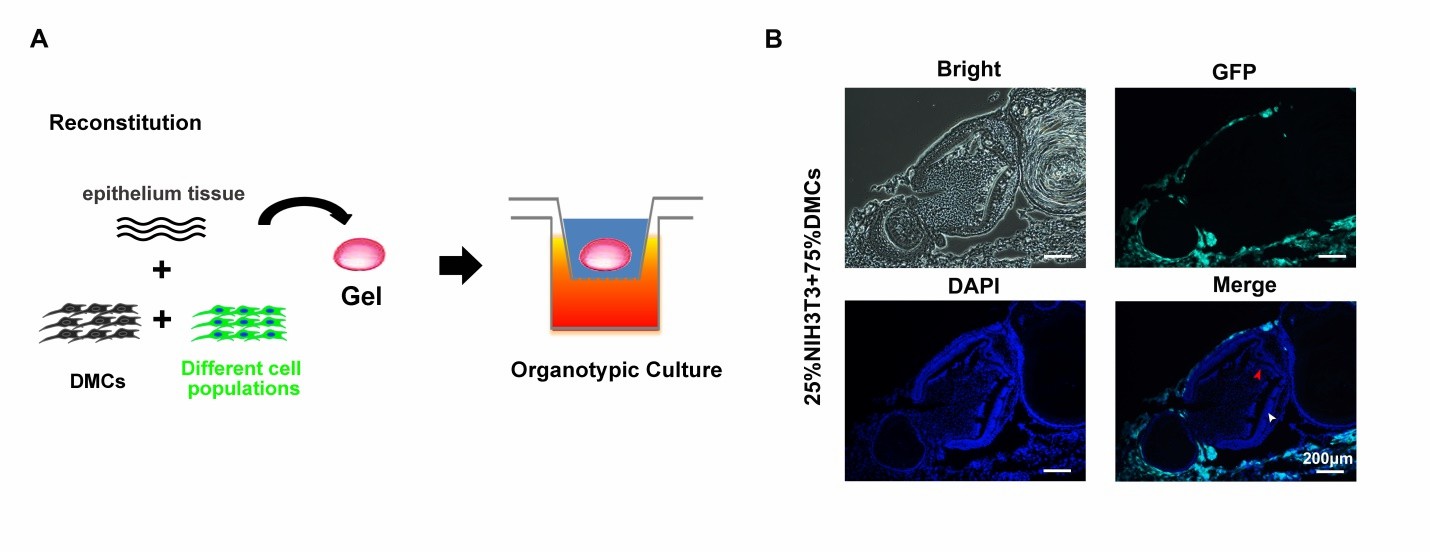 Fig. 1. Schematicof tooth germ reconstitution and NIH3T3 cells failure to contribute to tooth germ formation (Zhang M, Zhang XC, et al., 2020).
Fig. 1. Schematicof tooth germ reconstitution and NIH3T3 cells failure to contribute to tooth germ formation (Zhang M, Zhang XC, et al., 2020).
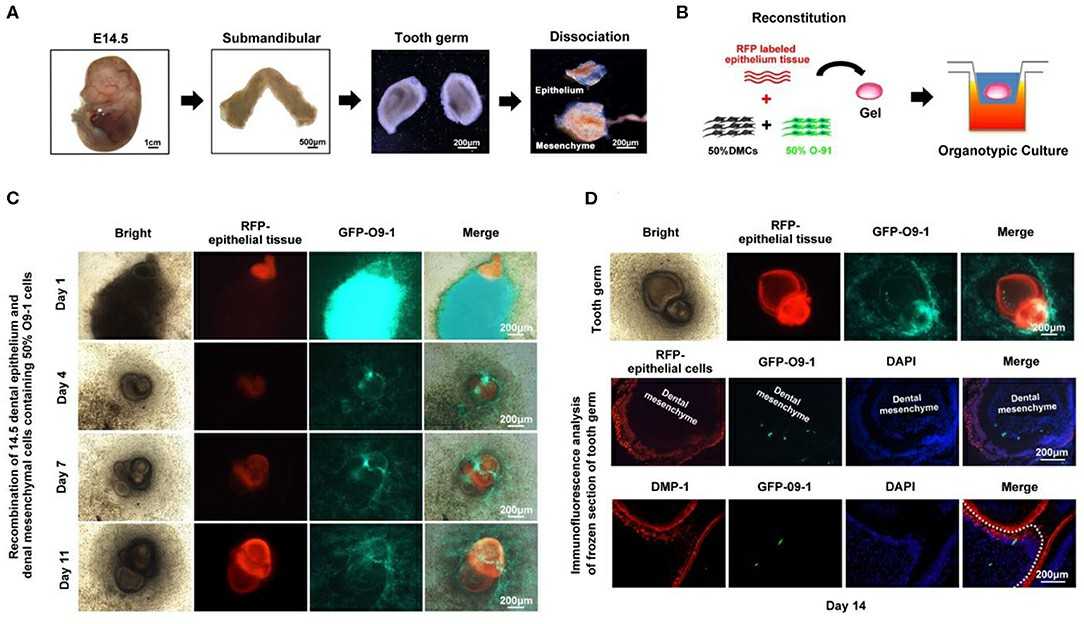 Fig. 2. O9-1 cells contribute to tooth germ formation in vitro (Zhang M, Zhang XC, et al., 2020).
Fig. 2. O9-1 cells contribute to tooth germ formation in vitro (Zhang M, Zhang XC, et al., 2020).
Overexpression of miR-129-5p and miR-340-5p Inhibits Cell Proliferation in MEPM and O9-1 Cells
Cleft lip and palate (CL/P) represent common birth defects that develop under the influence of genetic and epigenetic components combined with environmental elements. miRNAs are critical regulators of craniofacial development while also being linked to CL/P conditions. Yoshioka et al. explored whether suppressing specific miRNAs, particularly miR-124-3p and miR-340-5p, can prevent cleft palate induced by all-trans retinoic acid (atRA) in mice, offering a potential preventive strategy for CL/P.
To examine their role in orofacial clefts, they first treated primary MEPM cells, primary mouse embryonic lip mesenchymal (MELM) cells and O9-1 mouse cranial neural crest cells, an established mouse neural crest cell line, with either a miR-129-5p or miR-340-5p mimic. These mimics inhibited cell proliferation in palatal and O9-1 cells but not lip cells (Fig. 3A, B and Fig. 4A), indicating their role in palatal shelf growth. Next, to identify target genes regulated by miR-129-5p and miR-340-5p, we performed quantitative RT-PCR analysis for genes associated with CP in MEPM and O9-1 cells. miR-129-5p regulated Sox5, Tfap2a, Trp53, Zeb1, and others, while miR-340-5p regulated Chd7, Fign, and Tgfbr1 (Fig. 3C, D and Fig. 4B, C). Although miRNA inhibitors didn't affect proliferation in MEPM cells, they upregulated target genes, implying controlled gene regulation without promoting proliferation (Fig. 3E-G and Fig. 4D-F).
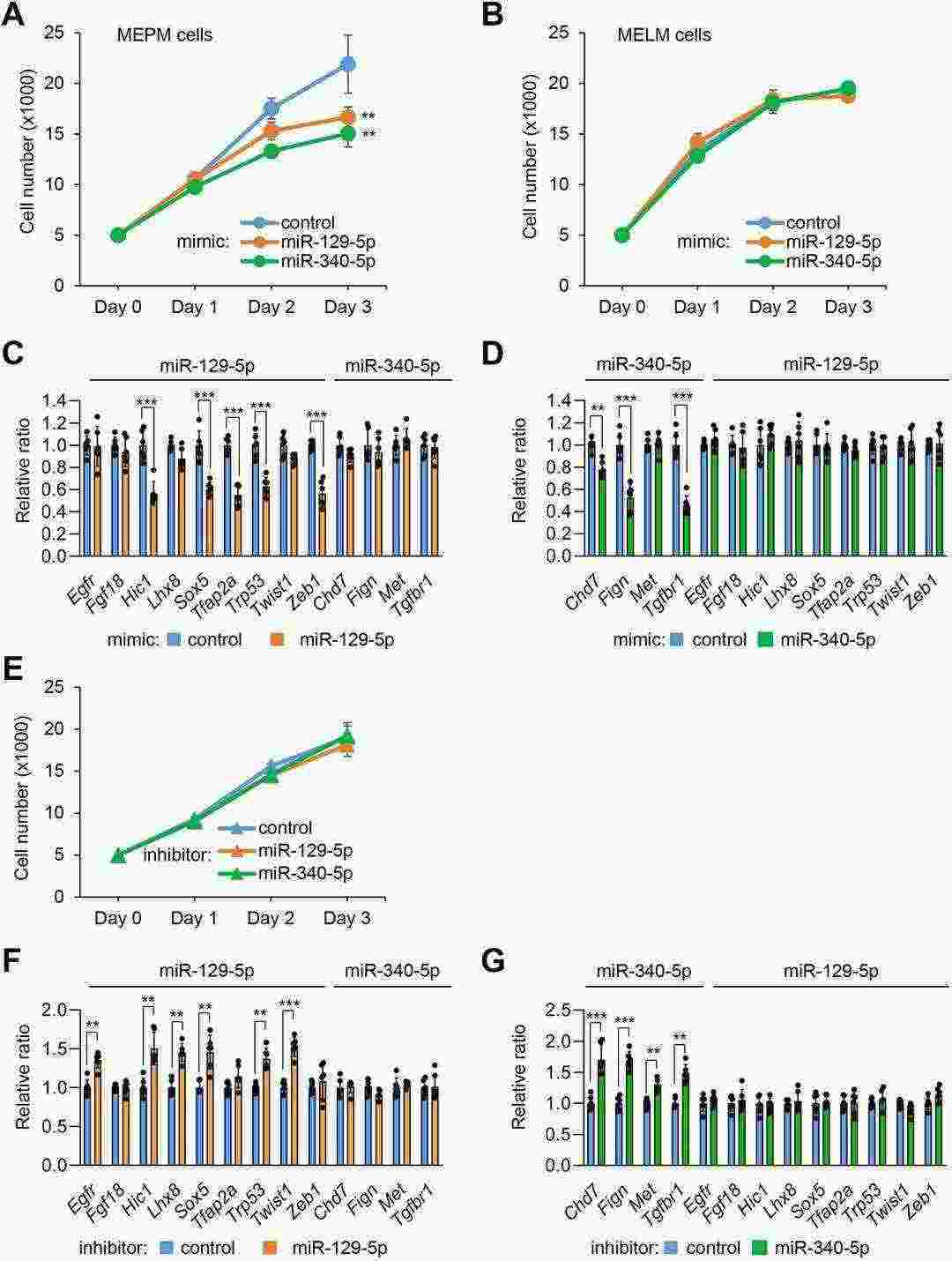 Fig. 3. Overexpression of miR-129-5p and miR-340-5p suppresses cell proliferation and gene expression in MEPM and MELM cells (Yoshioka H, Suzuki A, et al., 2022).
Fig. 3. Overexpression of miR-129-5p and miR-340-5p suppresses cell proliferation and gene expression in MEPM and MELM cells (Yoshioka H, Suzuki A, et al., 2022).
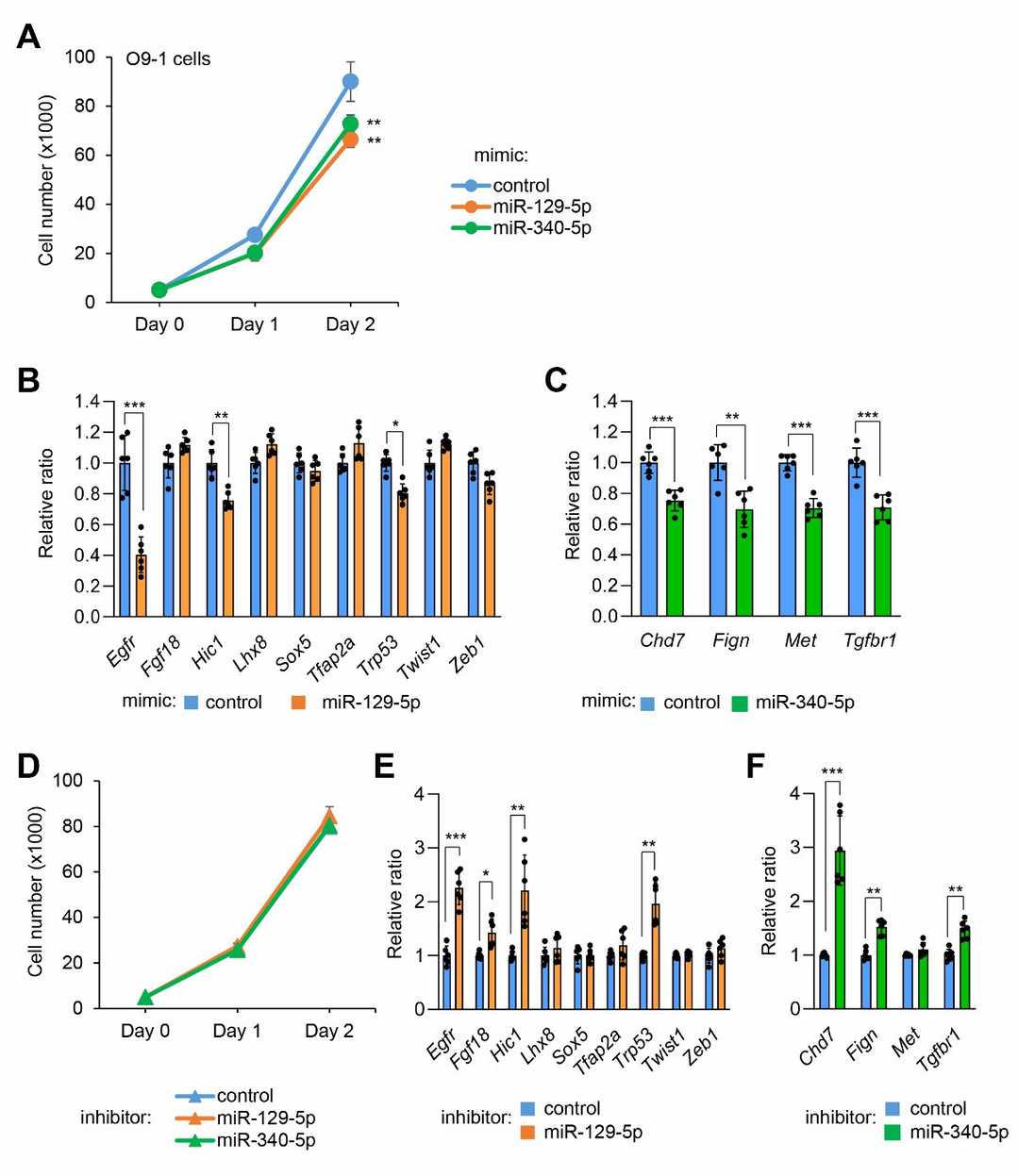 Fig. 4. Effect of miR-129-5p and miR-340-5p on cell proliferation and gene expression in O9-1 cells (Yoshioka H, Suzuki A, et al., 2022).
Fig. 4. Effect of miR-129-5p and miR-340-5p on cell proliferation and gene expression in O9-1 cells (Yoshioka H, Suzuki A, et al., 2022).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells