Human iPSC Line
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human induced pluripotent stem cell lines (Human iPSC Line) are stem cell lines obtained by somatic cell reprogramming technology, with pluripotency similar to embryonic stem cells (ESC) and can differentiate into cells of the three germ layers (ectoderm, mesoderm, and endoderm) in the human body. The major sources of cell lines are mainly somatic cells, including fibroblasts, peripheral blood mononuclear cells, keratinocytes, mesenchymal stem cells, neurons, neural stem cells, or other somatic cells. The iPSC generation method is based on somatic cell reprogramming, by introducing 4 important transcription factors (OCT4, SOX2, KLF4, c-MYC) or additional factors (such as NANOG, LIN28) to reprogram somatic cells into pluripotent stem cells.
After iPSCs are differentiated into specific cell types (cardiomyocytes, hepatocytes, neurons, etc.), they can be used for drug screening and toxicity testing. For example, iPSC-derived cardiomyocytes can be used to test the effects of drugs on cardiac function and the toxicity of drugs to specific types of cells. Meanwhile, iPSCs can also serve as an important tool for studying human early development, the mechanism of cell differentiation, and cell-cell interactions. We can also use iPSCs to construct three-dimensional organoids to simulate human organ development and function and study the regulation of transcription factors in the process of cell differentiation. In regenerative medicine, iPSCs can be further differentiated into neural stem cells, cardiomyocytes, hepatocytes, and other cell types for the treatment of diseases such as Parkinson's disease, heart failure, liver disease, etc.
 Fig. 1. Induced pluripotent stem cell (iPSC)-derived cellular models. The iPSC technology can be applied to derive cellular models of varying complexity, ranging from two-dimensional mono-cultures to three-dimensional multicellular assemblies (Cerneckis J, Cai H, et al., 2024).
Fig. 1. Induced pluripotent stem cell (iPSC)-derived cellular models. The iPSC technology can be applied to derive cellular models of varying complexity, ranging from two-dimensional mono-cultures to three-dimensional multicellular assemblies (Cerneckis J, Cai H, et al., 2024).
Generation and Maturation of Human Ipsc-Derived 3D Organotypic Cardiac Microtissues in Long-Term Culture
Cardiovascular diseases are a major global health concern, with existing models failing to fully replicate human heart complexity. Using induced pluripotent stem cells (iPSCs), Ergir et al. established a scaffold-free protocol to generate 3D human cardiac microtissues (hOCMTs).
Since 2D monolayer differentiation of iPSC-derived cardiomyocytes is well established, and these monolayers tend to delaminate into beating clusters, they first differentiated hiPSCs in a 2D system and tested their ability to form 3D cardiac aggregates without an external ECM scaffold. Cardiac differentiation was induced in confluent 2D hiPSC monolayers by modulating the WNT pathway with small molecules, as previously described (Fig. 1A). Briefly, mesoderm specification was achieved by transient WNT activation, followed by cardiac mesoderm differentiation through WNT inhibition. On day 7, insulin was added to support cell survival. On day 15, the monolayer was dissociated into single cells and seeded in ultra-low attachment plates to induce aggregate formation in the presence of a ROCK inhibitor. We used 2D monolayer cultures as controls. One day after switching to 3D culture, they observed spontaneous 3D microtissue formation without an ECM scaffold, which began beating after 48 h. To monitor this process, they repeated the experiment with hiPSCs expressing GFP-tagged cardiac troponin I. GFP-positive cardiomyocytes and non-GFP non-myocytes rearranged over time from a random distribution to more discrete regions (Fig. 1B). The microtissues' diameter increased from 0.9 ± 0.04 mm on day 21 to 1 ± 0.09 mm on day 42 (Fig. 1C). They continued to beat spontaneously in long-term culture, lasting at least until day 100.
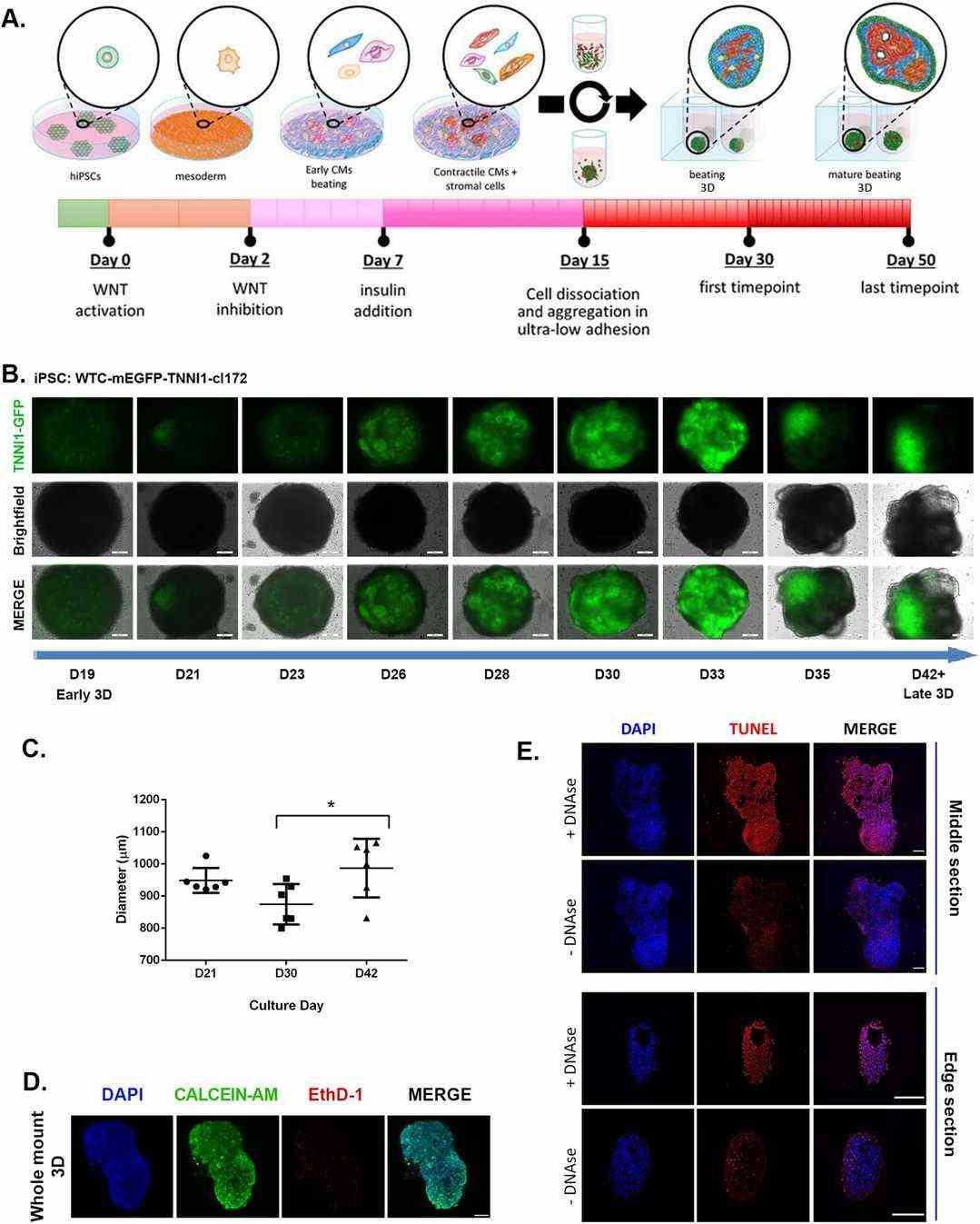 Fig. 1. Long-term human cardiac microtissues can be spontaneously generated by scaffold-free conditions in 3D (Ergir E, Oliver-De La Cruz J, et al., 2022).
Fig. 1. Long-term human cardiac microtissues can be spontaneously generated by scaffold-free conditions in 3D (Ergir E, Oliver-De La Cruz J, et al., 2022).
Efficient and Reproducible Generation of Human iPSC-Derived Cardiomyocytes and Cardiac Organoids in Stirred Suspension Systems
Human iPSC-derived cardiomyocytes (hiPSC-CMs) are crucial for cardiac disease modeling and regeneration, but challenges such as quality control, inter-batch consistency, cryopreservation, and scalability limit their reproducibility and clinical translation. Prondzyski et al. introduce a stirred suspension protocol using bioreactors and spinner flasks to produce hiPSC-CMs and cardiac organoids.
They first observed contraction in bCMs at differentiation day 5, compared to dd7 in mCMs and previously reported hiPSC-CM differentiations. To validate this, they differentiated hiPSCs with GFP fused to TNNI1 in the bioreactor and saw GFP expression in bCMs on dd5, in contracting areas at the edges of EBs (Fig. 2g). mCMs showed GFP expression on day 6 but did not visibly contract until dd7. RT-qPCR showed higher ACTN2 expression in dd5 bCMs than in mCMs (Fig. 2h). bCMs also had less inter-batch variation in ACTN2 levels at day 15 compared to mCMs (Fig. 2i). Vimentin (VIM) was lower in dd15 bCMs, though not significantly due to mCM variation (Fig. 2j). bCMs had higher expression of ventricular markers MYH7, MYL2, and MYL3 (Fig. 1k–m) and 83.4% MLC2v-positive cells by flow cytometry. Atrial markers MYL4 and MYL7 were higher or unchanged in bCMs (Fig. 2n, o). HCN4 was higher in some mCM batches with high variability (Fig. 2p). TNNT2 protein levels were significantly higher in dd15 bCMs than in mCMs.
 Fig. 2. Optimized stirred bioreactor cardiac differentiation protocol (Prondzynski M, Berkson P, et al., 2024).
Fig. 2. Optimized stirred bioreactor cardiac differentiation protocol (Prondzynski M, Berkson P, et al., 2024).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells