Strain C57BL/6 Mouse Embryonic Stem Cells with GFP
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
- Positive for alkaline phosphatase staining.
- Ability to form teratoma in severe combined immunodeficient (SCID) mice.
- ex
- Displaying normal diploid karyotype (40, XY or 40, XX) during extensive subcultivation.
- Ability to differentiate into cell types of all three germ layers in vitro and in vivo.
Upon receipt, if no dry ice is left in the package, thaw and use cells immediately.
If there is dry ice left in the package, store cells in liquid nitrogen immediately upon arrival.
When stored at the recommended sto
Embryonic stem cells from C57BL/6 mice are primarily derived from the inner cell mass (ICM) of mouse blastocysts. These cells show remarkable pluripotency which allows them to develop into any type of cell found in the body. In vitro, mouse embryonic stem cells grow alongside feeder cells such as mouse embryonic fibroblasts and have the ability to differentiate into various cell types including neurons and cardiomyocytes when exposed to appropriate induction conditions.
The defining characteristic of mouse embryonic stem cells makes them valuable tools in both regenerative medicine research and disease modeling studies. After injecting stem cells into nude mice they develop teratomas that display all three germ layers, thereby verifying their differentiation potential. These cell lines also provide ideal platforms for studying gene regulatory mechanisms. Researchers can track specific gene expression in real-time by observing changes in GFP fluorescence intensity. Furthermore, these cell lines are used to study the mechanisms of cell fate determination, including processes such as cell proliferation, differentiation, and apoptosis.
NanoTag - an IgG-free Method for Mapping DNA-protein Interactions—Indirect Profiling of H3K4me3 Sites in mESC
Protein-DNA interactions are essential for genome regulation. Traditional methods like ChIP-seq and CUT&Tag require IgG antibodies, which are limited by availability and production costs. Dimitriu et al. developed NanoTag, an IgG-free method using nanobodies to map DNA-protein interactions. NanoTag employs an anti-GFP nanobody-Tn5 transposase fusion to profile GFP-tagged proteins associated with chromatin.
To test NanoTag, they profiled TAF3, Nanog, and CTCF in GFP-tagged mouse embryonic stem cells (mESCs) (Fig. 1a, b, Fig. 2a, h). They compared NanoTag to CUT&Tag using the same cell lines. Both methods were performed in duplicate with 400,000 cells per sample. qPCR confirmed NanoTag enrichment for target regions. For TAF3, NanoTag showed high reproducibility and correlation with CUT&Tag data (Fig. 1c). Most TAF3-NanoTag peaks (76%) overlapped with CUT&Tag peaks (Fig. 1d) and were mainly at promoters. NanoTag coverage was similar to CUT&Tag at Hox genes (Fig. 1f). It also showed comparable sensitivity and low background noise (Fig. 1h, i). These results demonstrate NanoTag's effectiveness for profiling H3K4me3 sites in mESC using TAF3.
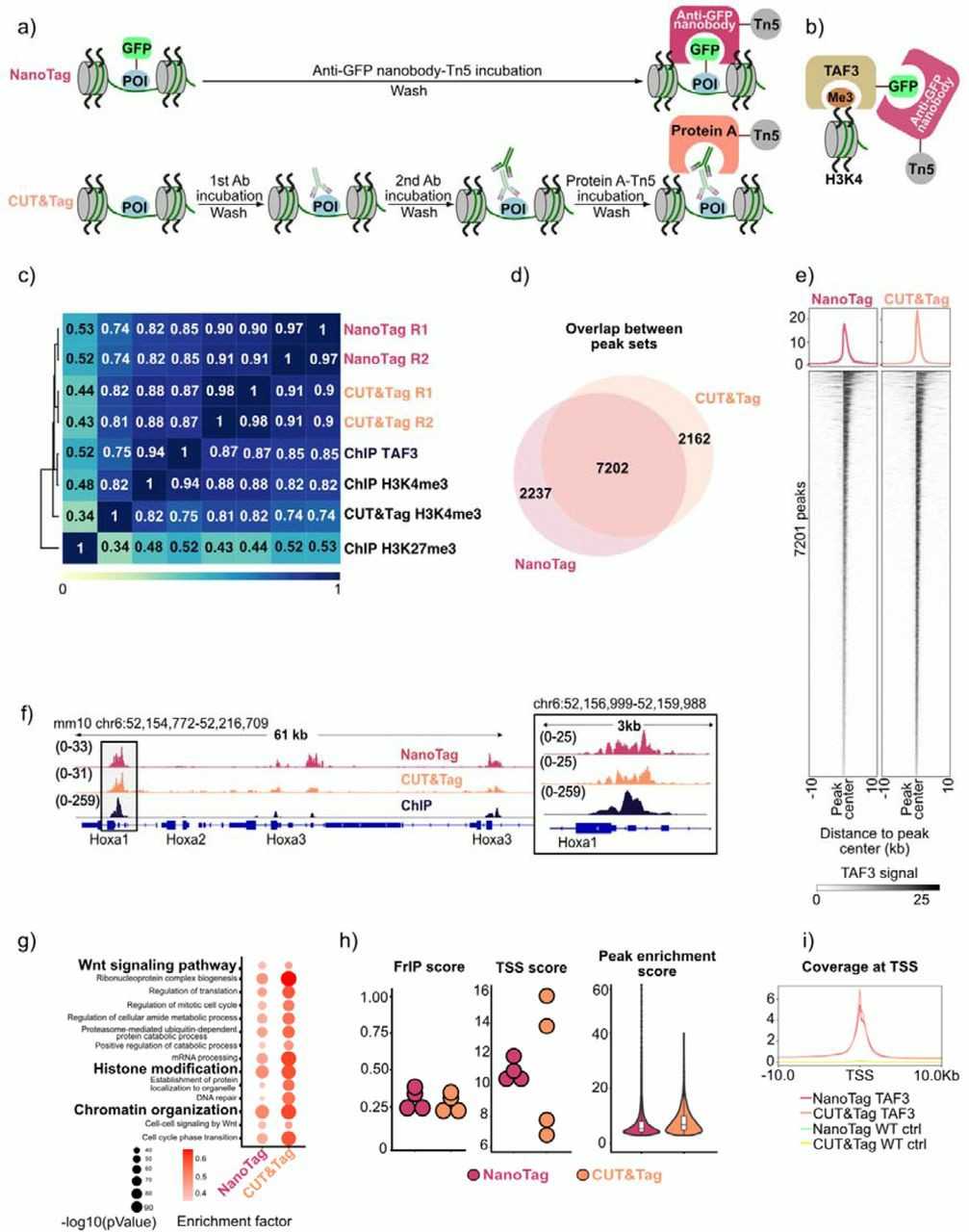 Fig. 1. Comparison of NanoTag and CUT&Tag workflow and profiling of TAF3 binding (Dimitriu MA, Arzate-Mejia RG, et al., 2024).
Fig. 1. Comparison of NanoTag and CUT&Tag workflow and profiling of TAF3 binding (Dimitriu MA, Arzate-Mejia RG, et al., 2024).
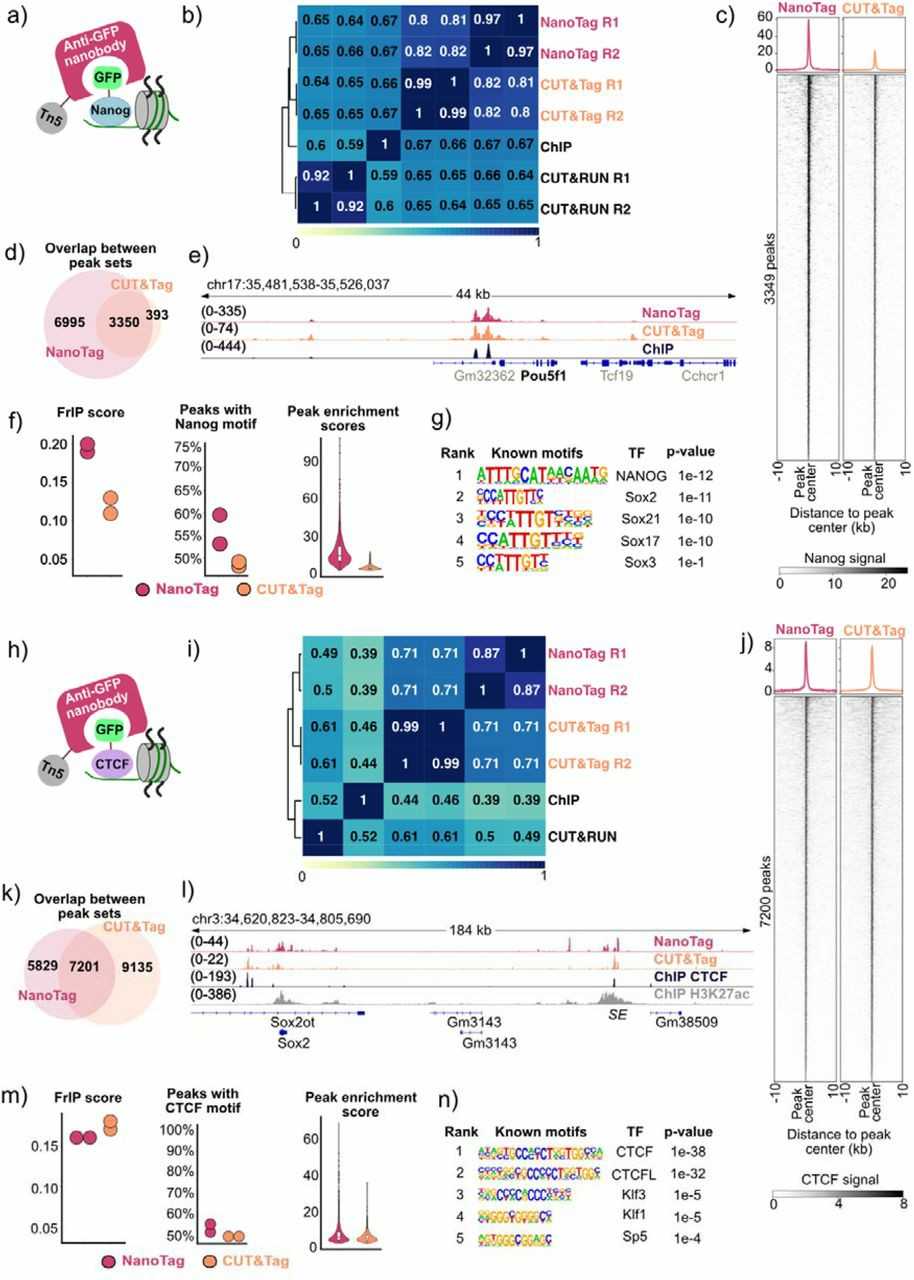 Fig. 2. Nanog and CTCF binding sites captured by NanoTag (Dimitriu MA, Arzate-Mejia RG, et al., 2024).
Fig. 2. Nanog and CTCF binding sites captured by NanoTag (Dimitriu MA, Arzate-Mejia RG, et al., 2024).
Gal1 Conditioning of SOD1G93A Microglia Decreases Microglia Mediated Motor Neuron Toxicity by Modulation of Microglial Activation in vitro
ALS stands as a neurodegenerative disorder defined by motor neuron degeneration and neuroinflammation. ALS pathogenesis results from contributions from both neuronal and non-neuronal cellular types. Baird et al. developed a combination therapy using AAV9-mediated SOD1 downregulation and galectin-1 (Gal1) expression. The method involves targeting motor neurons and astrocytes with AAV9 to knock down SOD1 and express Gal1, which indirectly targets microglia.
Through transfection of HEK293 cells with a Gal1 plasmid and a red fluorescent protein (RFP) plasmid as a control the study investigated how Gal1 influenced ALS microglia. Every 24 hours researchers collected supernatants for a total of 72 hours. Gal1 overexpression in HEK293 cells was confirmed by immunocytochemistry (Fig. 3A), and increased Gal1 secretion was verified by ELISA on supernatants. Microglia from SOD1G93A mice and controls were conditioned with Gal1 or RFP supernatants for 3 days before co-culture with GFP-positive mouse motor neurons (Fig. 3B). Motor neurons were derived from mouse embryonic stem cells expressing GFP driven by the Hb9 promoter, sorted for GFP+ cells, and plated at 6,000 cells per well. Microglia were added at 35,000 cells per well. After 72 hours, motor neuron survival was significantly lower with SOD1G93A microglia than with WT microglia (Fig. 3C). However, Gal1 preconditioning rescued motor neuron death (Fig. 3D and 3E).
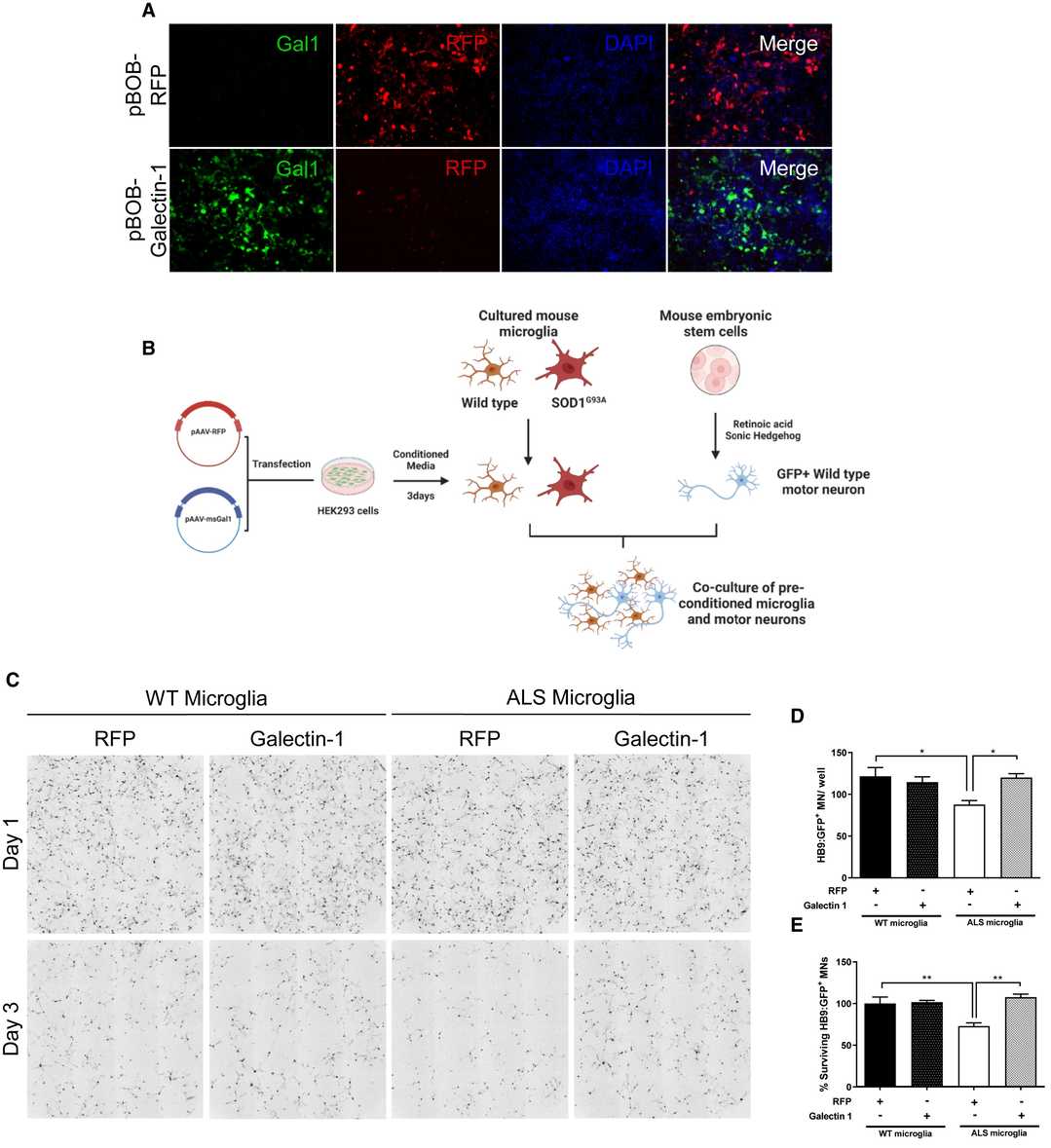 Fig. 3. Gal1 conditioning of SOD1G93A microglia reduces microglia-mediated motor neuron toxicity (Baird MC, Likhite SB, et al., 2024).
Fig. 3. Gal1 conditioning of SOD1G93A microglia reduces microglia-mediated motor neuron toxicity (Baird MC, Likhite SB, et al., 2024).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells