- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells
Immortalized Human Podocytes
Cat.No.: CSC-I1918Z
Species: Homo sapiens
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20°C.
Mature podocytes are highly specialized, terminally differentiated cells that play a key role in glomerular filtration. They surround the glomerular capillaries to form a network of interdigitating foot processes (FP) that are interconnected by specialized adherens junction called the slit diaphragm. When the primary filtrate passes through the slit diaphragm, high molecular weight molecules such as plasma proteins are kept back, making the podocytes an essential component of glomerular filtration.
Indeed, glomerular diseases account for approximately 90% of end-stage renal disease in the US, and most glomerulopathies are characterized by podocyte injury, resulting in fibrosis, proteinuria and loss of kidney function. Due to their limited regenerative potential, mature podocytes are especially vulnerable to a variety of toxic insults within the functional setting of a complex glomerulus under physiologic conditions (blood filtration, blood pressure, etc.). Given this, robust, reproducible and sensitive assessment of toxic stimuli to which human podocytes are exposed in vitro is critical to understanding human glomerulopathies and predicting glomerulotoxicity, either for enhancing mechanistic understanding or for medium-high-throughput screening.
Primary cells are unsuitable for screening purposes as well as mechanistic studies, as they dedifferentiate rapidly and are not readily available in sufficient quantities with reproducible quality. Immortalized podocytes allow for an in vitro process of maturation analogous to the development and maturation of podocytes in vivo. The result is a homogenous, stable cell source that shows expression of key antigenic markers of differentiated podocytes in vivo. These include the novel podocyte proteins nephrin, podocin, CD2AP and synaptopodin. This robust cell line has been extensively characterized and validated, and is used worldwide.
Characterization of Immortalized Human Podocytes Infected with Lentivirus as An In Vitro Model of Viral Infection-Associated Podocytopathy
A large number of studies have shown the association of kidney disease with viral infections in the body. Viral infections cause kidney injury in two manners, the systemic inflammation (cytokine storm) and the direct infection of kidney cells. Concerning direct viral infection of podocytes, the mechanism underlying virus-induced podocyte injury remains largely unknown and requires effective experimental models to facilitate its study. Here, we performed molecular characterization of immortalized human podocyte cell line (HPC) infected with lentivirus by RNA-seq. Bioinformatics analysis revealed a strong innate immune response in the cells, including interferon production and signaling. Meanwhile, activations of ferroptosis pathway and TNF-alpha signaling were also found, consistent with an impaired viability of the cells. Lentiviral infection also upregulated expression of APOL1 as observed in patients with HIV associated nephropathy (HIVAN) and diabetic nephropathy (DN). Thus, the lentivirus-infected HPC cells represent a useful in vitro model of viral infection-associated podocytopathy.
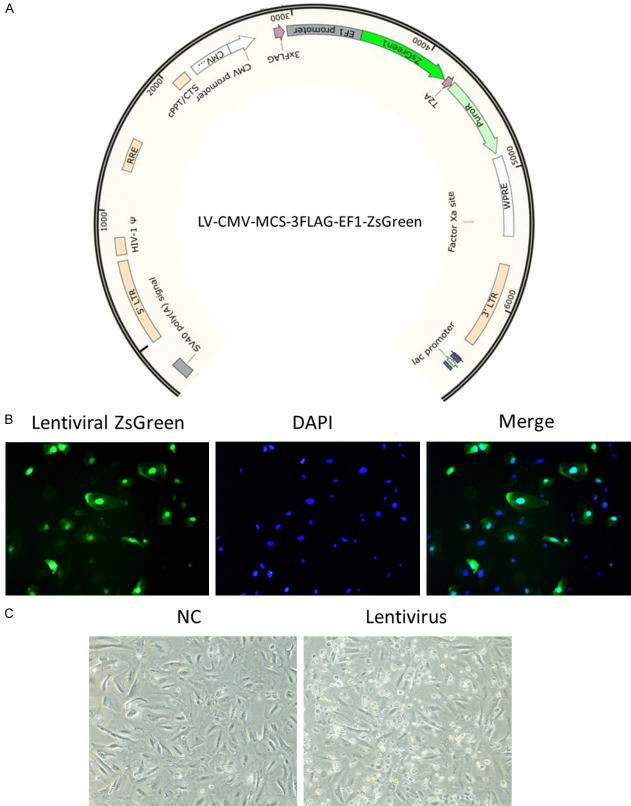 Fig. 1. Infection of lentivirus in immortalized human podocytes induced cell death (Yu, Peng, et al. 2024).
Fig. 1. Infection of lentivirus in immortalized human podocytes induced cell death (Yu, Peng, et al. 2024).
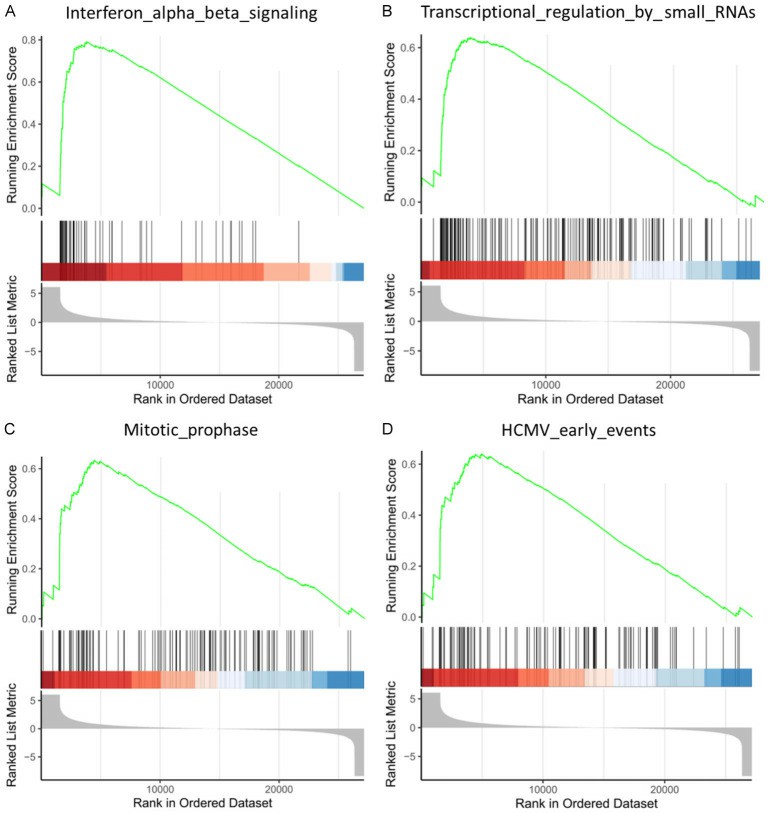 Fig. 2. GSEA analysis of the differentially expressed genes in control and retrovirus-infected HPC cells (Yu, Peng, et al. 2024).
Fig. 2. GSEA analysis of the differentially expressed genes in control and retrovirus-infected HPC cells (Yu, Peng, et al. 2024).
Proteomic Changes Induced by the Immunosuppressant Everolimus in Immortalized Human Podocytes
mTOR inhibitors (mTOR-Is) may induce proteinuria in kidney transplant recipients by damaging podocytes. However, the mechanism has only been partially defined. Total cell lysates and supernatants of immortalized human podocytes treated with different doses of everolimus (EVE) (10, 100, 200, and 500 nM) for 24 h were subjected to mass spectrometry-based proteomics. Support vector machine and partial least squares discriminant analysis were used for data analysis. We identified more than 7000 differentially expressed proteins involved in several pathways, including kinases, cell cycle regulation, epithelial–mesenchymal transition, and protein synthesis, according to gene ontology. Among these, after statistical analysis, 65 showed an expression level significantly and directly correlated with EVE dosage. Polo-Like Kinase 1 (PLK1) content was increased, whereas osteopontin (SPP1) content was reduced in podocytes and supernatants in a dose-dependent manner and significantly correlated with EVE dose. This study helps to understand not only the biological basis of mTOR-Is therapeutic effects but also the possible mechanisms underlying proteinuria.
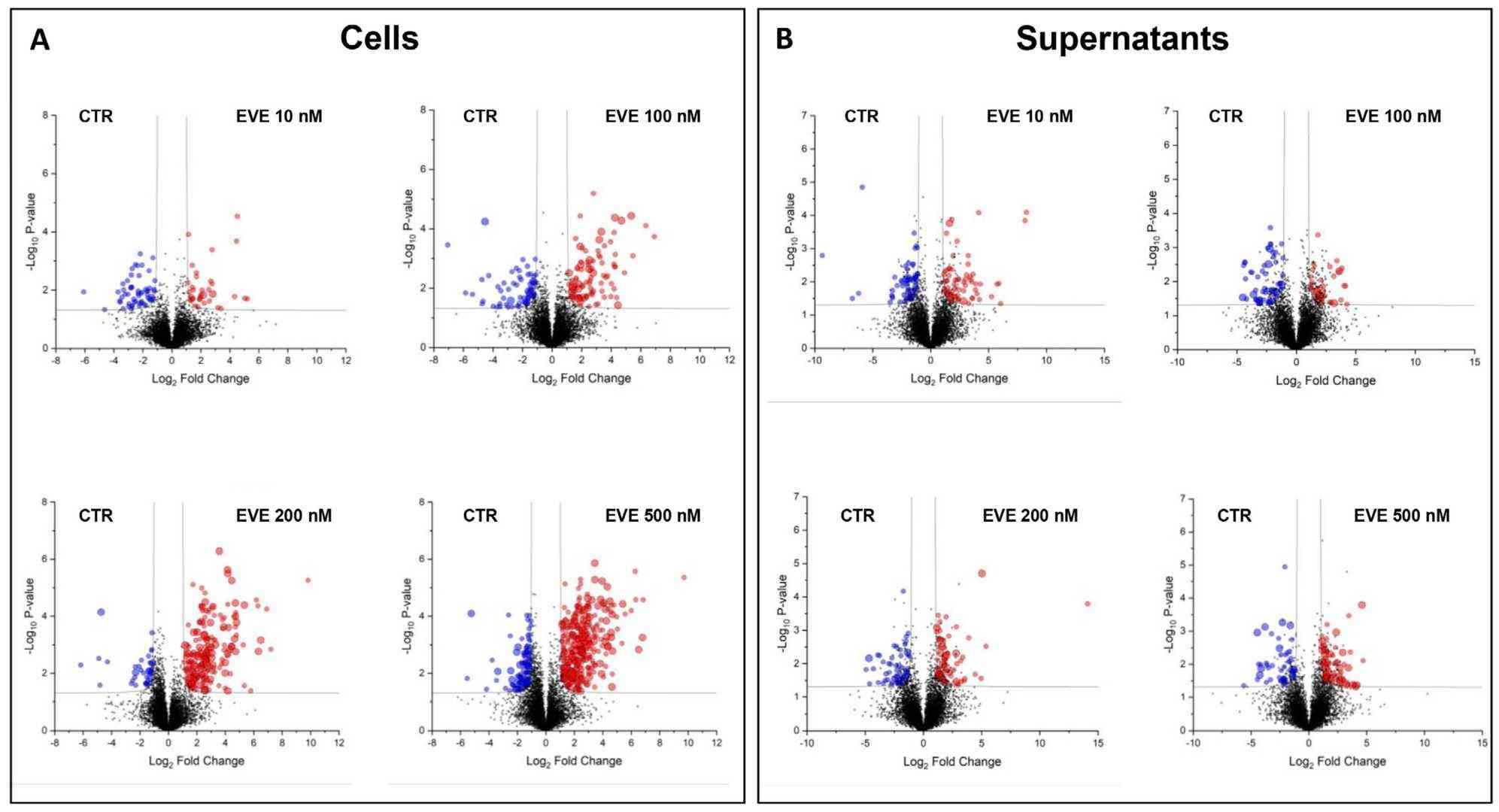 Fig. 3. Volcano plots of the comparison between untreated and everolimus (EVE)-treated (A) podocytes and (B) their supernatants (Bruschi, Maurizio, et al. 2024).
Fig. 3. Volcano plots of the comparison between untreated and everolimus (EVE)-treated (A) podocytes and (B) their supernatants (Bruschi, Maurizio, et al. 2024).
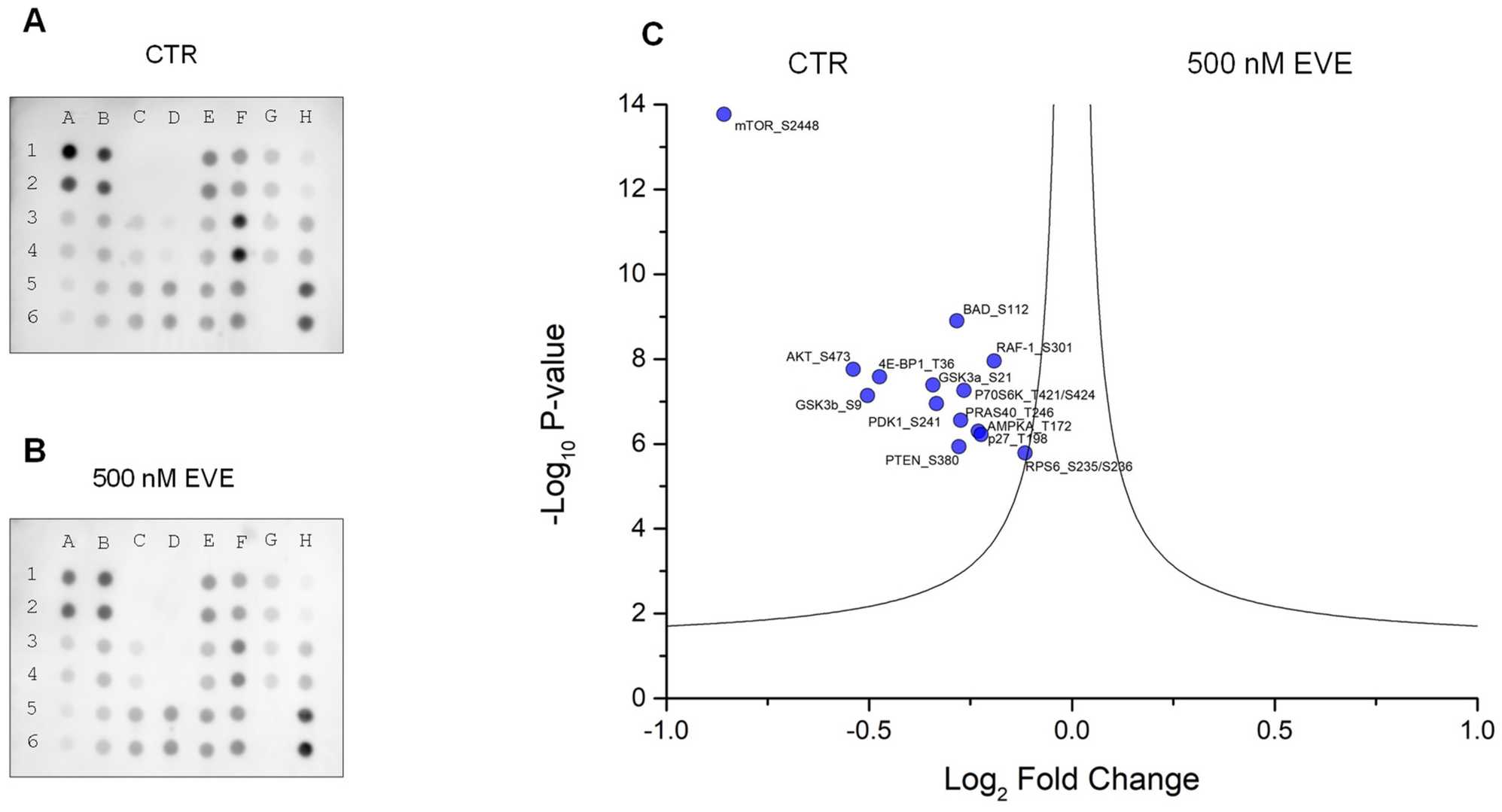 Fig. 4. AKT-mTOR phosphorylation pathway profiling array (Bruschi, Maurizio, et al. 2024). (A) Podocytes untreated (CTR). (B) Podocytes treated with 500 nM everolimus (EVE).
Fig. 4. AKT-mTOR phosphorylation pathway profiling array (Bruschi, Maurizio, et al. 2024). (A) Podocytes untreated (CTR). (B) Podocytes treated with 500 nM everolimus (EVE).
These cells are ideal for a wide range of renal research applications, including studies of kidney function, disease modeling for conditions like nephrotic syndrome, drug testing and nephrotoxicity assessments, and genetic studies focused on kidney health.
Immortalized Human Podocytes (Cat No.: CSC-I1918Z) provide a consistent and reliable platform, offering reproducibility and reduced variability that primary cells often cannot achieve due to their limited lifespan and donor-related differences. This makes them suitable for long-term and large-scale studies.
Immortalized Human Podocytes (Cat No.: CSC-I1918Z) stand out because they maintain critical functions and characteristics of primary podocytes, such as expression of key markers and the ability to form foot processes in culture, combined with the capacity to be cultured over extended periods.
To place an order or request more information, please contact us through our customer service channels. We are dedicated to providing you with detailed information and excellent support throughout the purchasing process. Advance your kidney research with the reliability and versatility of Immortalized Human Podocytes from Creative Bioarray. Order now to elevate your research and drive scientific breakthroughs!
Ask a Question
Write your own review