- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells
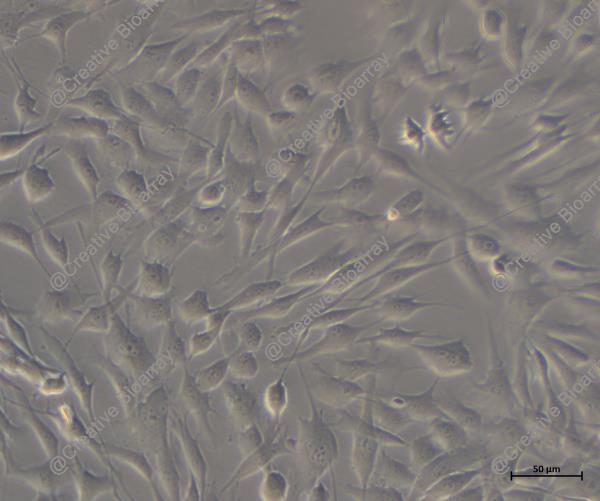
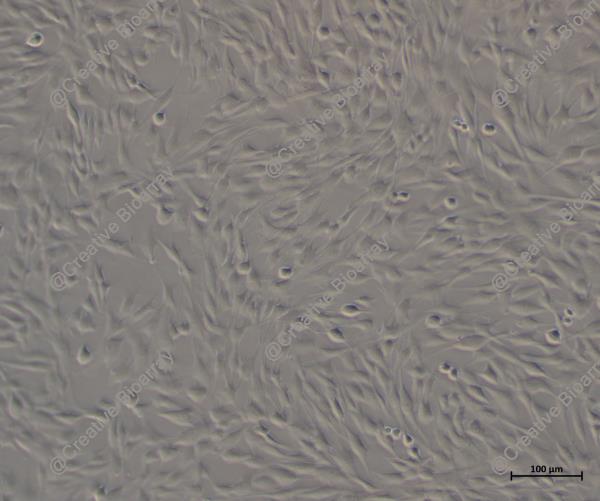
Immortalized Human Colonic Epithelial Cells
Cat.No.: CSC-I1915Z
Species: homo sapiens
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Q & A
- Customer Review
Note: Never can cells be kept at -20°C.
These are human colonic epithelial cells that have been immortalized to enable continuous proliferation while maintaining essential characteristics of normal colonic epithelial cells. They serve as a robust model for studying various aspects of intestinal biology and disease.
These cells are versatile and can be used in several research areas, including:
Gastrointestinal physiology and pathophysiology.
Colorectal cancer studies.
Drug absorption and metabolism research.
Intestinal barrier function and permeability studies.
Investigations of inflammatory bowel diseases (IBD).
Ask a Question
Write your own review