DOHH-2
Cat.No.: CSC-C0219
Species: Homo sapiens (Human)
Source: Pleural Effusion
Morphology: cells grow as single cells or in clumps in suspension
Culture Properties: suspension
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
- Documents
Immunology: CD3 -, CD10 +, CD13 -, CD19 +, CD20 +, CD34 -, CD37 +, CD38 +, CD80 +, CD138 -, HLA-DR +, sm/cyIgG +, sm/cyIgM -, sm/cykappa -, sm/cylambda +
Viruses: ELISA:
DOHH-2 cells are a cell line derived from the pleural effusion of a 60-year-old man diagnosed with refractory immunoblastic B-cell lymphoma that had progressed from follicular centroblastic/centrocytic lymphoma. The cells were originally established in 1990 and have since been used in various studies related to lymphoma, cancer biology, and molecular genetics.
One of the defining characteristics of DOHH-2 cells is the presence of the t (14;18) chromosomal translocation, leading to the formation of the IGH-BCL2 fusion gene. This translocation is commonly found in B-cell lymphomas and results in the overexpression of the BCL2 protein, which plays a role in inhibiting apoptosis (programmed cell death). The expression of BCL2 mRNA in DOHH-2 cells suggests their potential contribution to the development and progression of lymphoma.
It is worth noting that the original culture of DOHH-2 cells was a mixture of Epstein-Barr virus (EBV)-negative and EBV-positive cells. Researchers isolated and clonally expanded the EBV-negative cells for further study and establishment of the cell line. This feature provides researchers with the opportunity to investigate the impact of EBV infection on lymphoma development and progression.
EBV activates NKL homeobox genes in DOHH-2 Cells
NKL homeobox genes encode developmental transcription factors regulating basic processes in cell differentiation. EBV infects B-cells and influences the activity of signaling pathways. Therefore, EBV infection impacts the pathogenesis and the outcome of B-cell malignancies including Hodgkin lymphoma and diffuse large B-cell lymphoma (DLBCL).
Limited dilution of DLBCL-derived cell line DOHH-2 was performed to end up with pure EBV-positive and EBV-negative subclones. Subsequent FISH and PCR analyses confirmed the presence and absence of EBV in all cells of the isolated subclones (Fig. 1A and 1B). To determine the latency program operating in EBV-positive DOHH-2 subclones, an RT-PCR analysis of selected EBV-encoded genes (Fig. 1C) was performed. Quantification of CD21 expression by RQ-PCR demonstrated high levels in EBV-positive DOHH-2 cells while expression in EBV-negative DOHH-2 cells was barely detectable (Fig 1D). In contrast, the expression levels of the B-cell receptor cofactor CD19 were similar in both DOHH-2 subclones (Fig. 1D).
Expression profiling data generated from EBV-positive and EBV-negative DOHH-2 subclones showing the top-1000 differentially expressed genes reflected their deregulation (Fig. 2A). In addition, the differing expression levels of these genes were confirmed by RQ-PCR. The EBV-positive subclone expressed significantly decreased levels of BCL6, BACH2, and IL4R (Fig. 2B) while the genes for IRF4, MIR155HG, and PRDM1 showed elevated transcript levels (Fig. 2C). However, RQ-PCR and Western blot analyses showed no significant difference between EBV-positive and EBV-negative subclones (Fig. 2C). RQ-PCR analysis of these genes in EBV-positive/negative DOHH-2 cells demonstrated significant differences for HLX, NKX6-3 and MSX1 while the levels of HHEX were similar in both subclones (Fig. 2D). As compared to primary hematopoietic cells/tissues, EBV-positive DOHH-2 cells expressed enhanced levels of HLX, resembling those in bone marrow and exceeding the levels in lymph nodes and spleen (Fig. 2E).
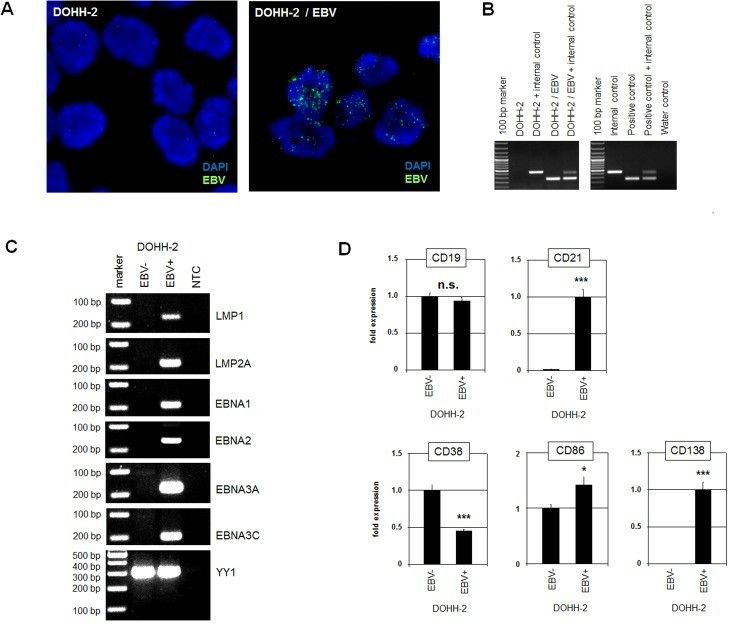 Fig. 1 Characterization of EBV-positive/negative DOHH-2 subclones. (Nagel S, et al., 2019)
Fig. 1 Characterization of EBV-positive/negative DOHH-2 subclones. (Nagel S, et al., 2019)
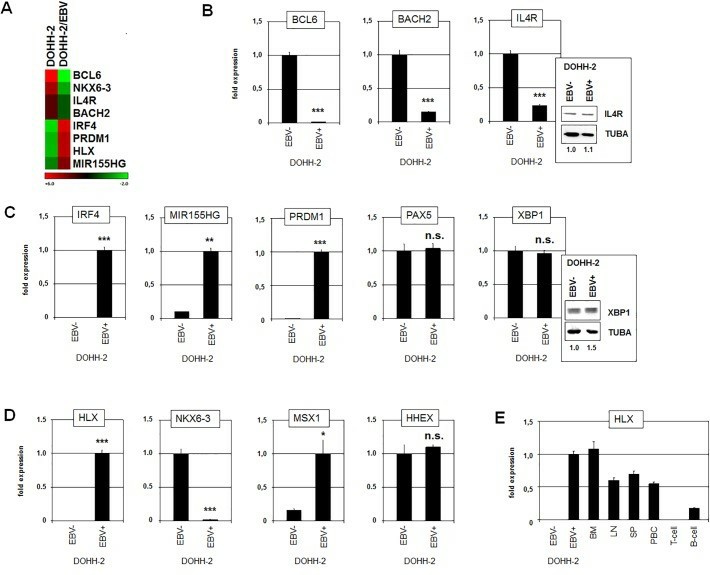 Fig. 2 Gene expression analyses in EBV-positive/negative DOHH-2 subclones. (Nagel S, et al., 2019)
Fig. 2 Gene expression analyses in EBV-positive/negative DOHH-2 subclones. (Nagel S, et al., 2019)
Targeting B-cell malignancies with anti-CD37 radioimmunoconjugate 177Lu-NNV003
CD37 has been used as a target for naked antibodies and two antibody-drug conjugates. Furthermore, the next generation RIC is the murine version of 177Lu-NNV003 and is currently in clinical trials for relapsed/refractory lymphomas.
177Lu-NNV003 showed an antiproliferative effect against the cell lines tested (Fig. 3). The biodistribution of 177Lu-NNV003 was performed in NSG mice with s.c. MEC-2 xenografts and in CB17 SCID mice with s.c. DOHH-2 xenografts (Fig. 4). 177Lu-NNV003 reached a maximum uptake of 45% of injected activity/g in MEC-2 tumors after three days and 15% in DOHH-2 tumors after two days. The injected activity/g was approximately 12% for DOHH-2 and 10% for MEC-2 7 days after injection. After initial distribution to normal organs, no redistribution of the radionuclide was observed. The absorbed radiation dose after injection of 100 MBq/kg 177Lu-NNV003 in DOHH-2 tumors was 4.6 Gy and lower than 1.2 Gy in all of the normal tissues, except in blood (Fig. 5).
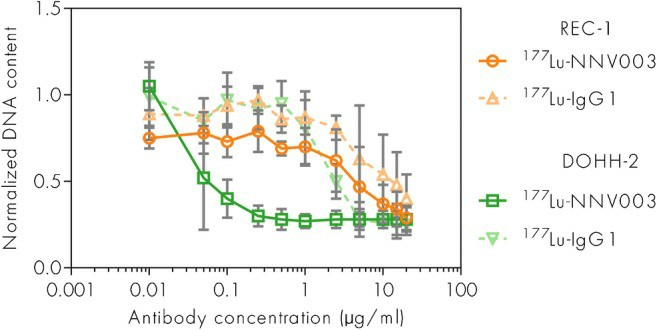 Fig. 3 Anti-tumor effect of 177Lu-NNV003 in REC-1 and DOHH-2 cells. (Lin JT, et al., 2022)
Fig. 3 Anti-tumor effect of 177Lu-NNV003 in REC-1 and DOHH-2 cells. (Lin JT, et al., 2022)
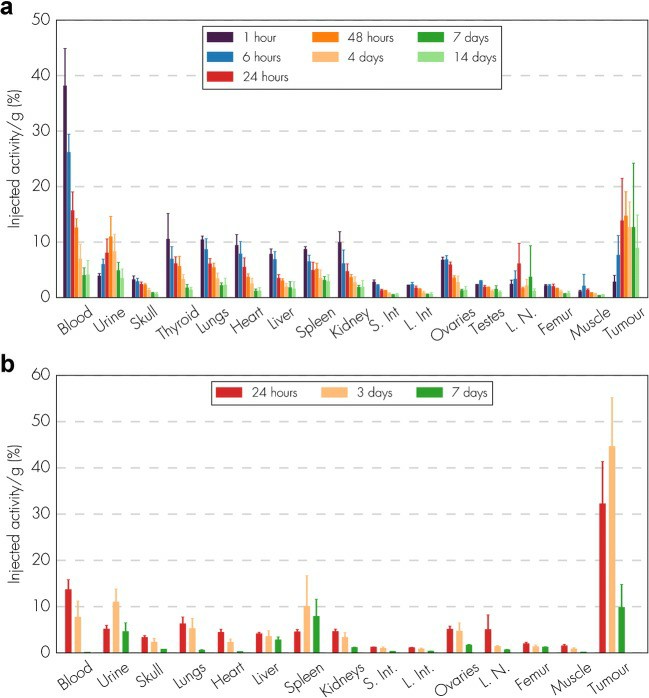 Fig. 4 Biodistribution of 177Lu-NNV003. (Lin JT, et al., 2022)
Fig. 4 Biodistribution of 177Lu-NNV003. (Lin JT, et al., 2022)
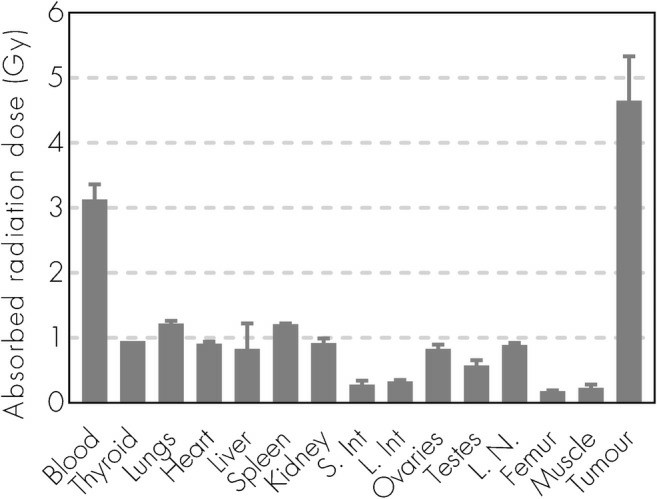 Fig. 5 Absorbed radiation dose (Gy) to organs and tumor for CB17 SCID mice with DOHH-2 s.c. xenografts injected with 177Lu-NNV003. (Lin JT, et al., 2022)
Fig. 5 Absorbed radiation dose (Gy) to organs and tumor for CB17 SCID mice with DOHH-2 s.c. xenografts injected with 177Lu-NNV003. (Lin JT, et al., 2022)
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells