Human Mesenchymal Stem Cells-bone marrow (HMSC-bm)
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
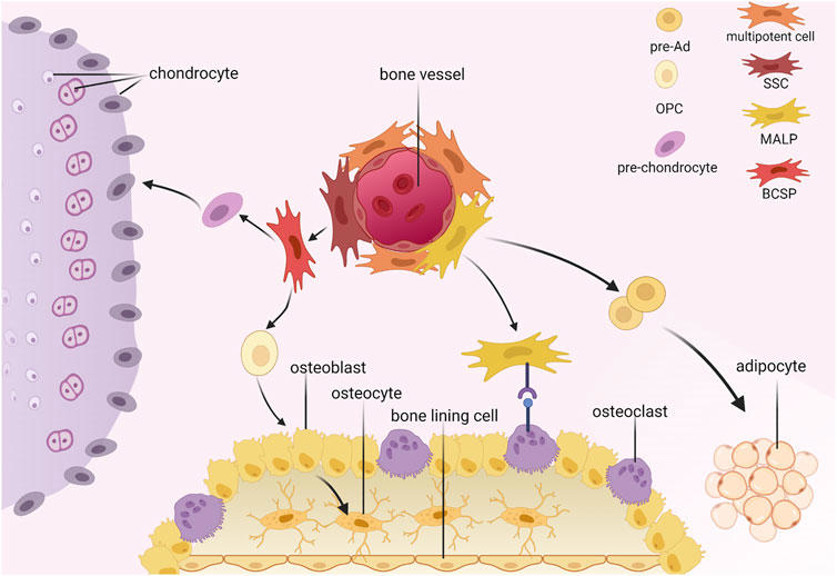
Human Mesenchymal Stem Cells-bone marrow (HMSC-bm) are derived from human bone marrow, which is a vital hematopoietic tissue in the human body and is also rich in mesenchymal stem cells (MSCs). These cells play a significant role in the bone marrow microenvironment. HMSC-bm cells have a spindle-shaped morphology, with a few cells possibly exhibiting a myofibroblast-like appearance. HMSC-bm cells exhibit an adherent growth mode under in vitro culture conditions. HMSC-bm cells are multipotent, with the potential to differentiate into various cell types, including adipocytes, chondrocytes, osteoblasts, tendinocytes, and myocytes. Additionally, HMSC-bm cells possess immunomodulatory properties and can modulate inflammatory responses by secreting cytokines such as IL-6, IL-8, and IL-10.
HMSC-bm cells can be utilized for fundamental scientific research as well as clinical practices. In basic research, they are often used to investigate cell differentiation, tissue regeneration, and disease mechanisms. In clinical settings, these cells can be used in tissue engineering, cell therapy, and regenerative medicine, such as using HMSC-bm cells to repair damaged tissue, promote tissue regeneration, and improve tissue function.
Therapeutic Effects of MSCs on Lung Injury and Inflammatory Response
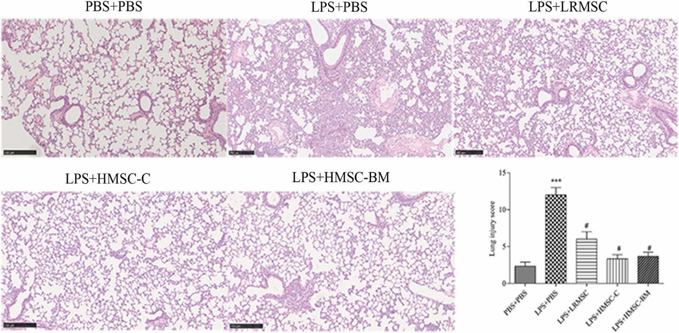
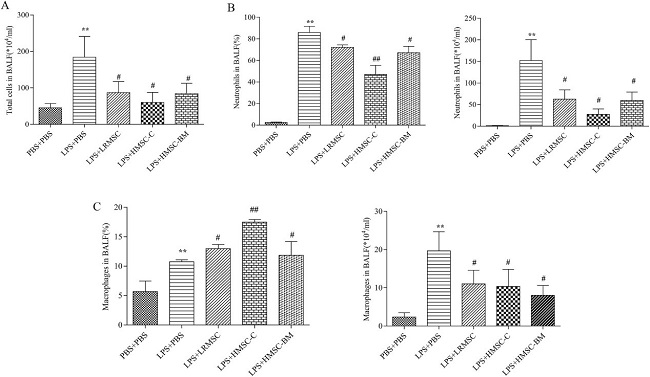
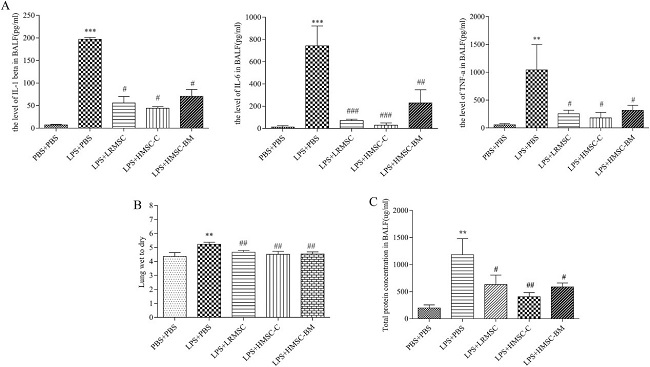
Mesenchymal stem cells (MSCs) can effectively attenuate acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). However, the optimal source of MSCs for cell-based therapy remains unknown. Here, Wang’s team compared the therapeutic effect of rat lung resident MSC (LRMSC), human chorion-derived MSC (HMSC-C) and human bone marrow derived MSC (HMSC-BM) in LPS induced ALI in mice.
Intratracheal instillation of LPS caused a robust inflammatory response in the lung (Fig. 1A and 1B). Administration of LRMSC, chorion-derived MSCs and bone marrow derived MSCs all decreased the inflammatory cells infiltration and the interstitial thickening induced by LPS (Fig. 1C-E). They also found that administration of LRMSC, chorion-derived MSCs and bone marrow derived MSCs significantly reduced the lung injury score and the effect of chorion-derived MSCs and bone marrow derived MSCs were stronger than that of LRMSC (Fig. 1F). Moreover, all these three kinds of MSCs attenuated the influx of inflammatory cells (Fig. 2) and reduced the secretion of the inflammatory cytokines IL-1β,IL-6, and TNF-α in the injured alveolus, and the effect of chorion-derived MSCs is best (Fig. 3A).
Hyaluronan-Based Hydrogel Delivering Glucose to Mesenchymal Stem Cells Intended to Treat Osteoarthritis
Mesenchymal stem cell (MSC) therapy holds promise for osteoarthritis (OA) treatment by secreting growth factors and extracellular matrix molecules to regenerate cartilage. However, MSCs injected into the non-vascularized joint environment quickly die due to lack of energy supply. To address this, a glucose-bound hyaluronic acid (HA) derivative hydrogel was developed to provide a controlled glucose supply to MSCs, enhancing their survival and therapeutic efficacy in nutrient-deficient OA joints.
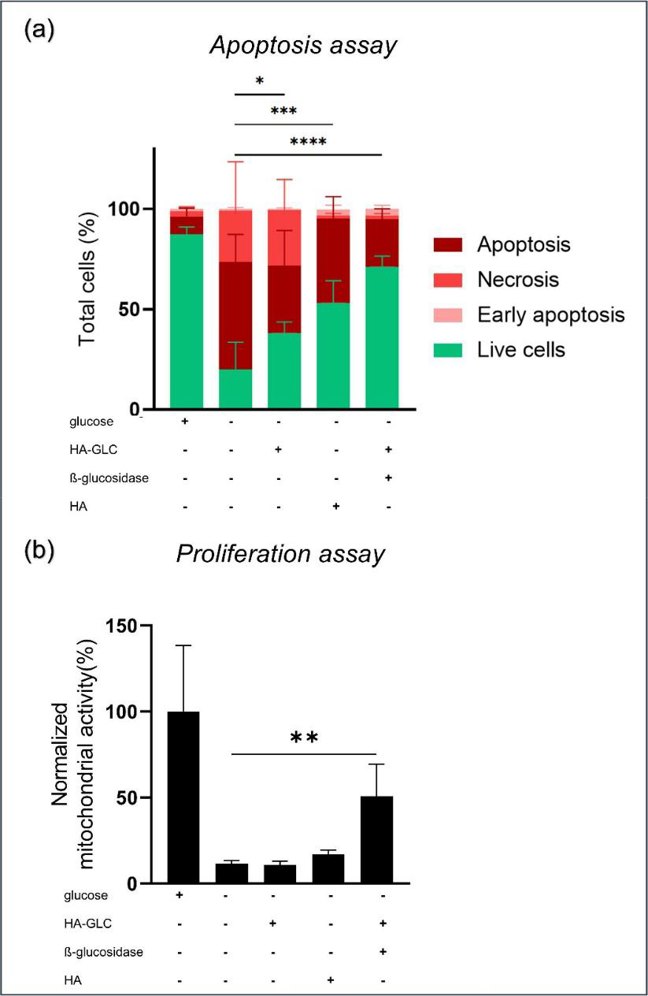
In this work, Gonzalez-Fernandez et al. investigated how the cell behaves under glucose starvation in terms of apoptosis and proliferation and they were hypothesizing that HA-GLC could reverse apoptosis and restore normal proliferation to glucose-free media. MSCs are deprived of nutrients and glucose in a non-vascularized environment such as in joints. To create a similar environment, they kept the cells in a medium that had no serum or glucose components (SD4). Flow cytometry was used to confirm MSC markers (CD73+, CD90+, HLA I+, HLA II−) to confirm that the cells did not differentiate during the treatment. The viability of MSCs under different conditions was determined by apoptosis/necrosis assay (Annexin V/PI double-staining) (Fig. 4a). The control conditions showed glucose is vital for cell survival. Adding HA-GLC to glucose-free medium significantly reduced cell death. To determine whether HA alone has an effect on cell viability, HA was added to glucose-free media. In this condition, 53% of the MSCs survived after 72 h. This result suggested that HA itself could be a sufficient energy source for the cells. This was consistent with the previously reported ability of HA to nurture stem cells.
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells