
Immortalized Mouse Renal Proximal Tubule Cells
Cat.No.: CSC-I9243L
Species: Mus musculus
Source: Kidney
Morphology: Cobblestone
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT003 HT? Lenti-SV40T Immortalization Kit
2) qPCR analysis on SV40T expression.
3) Morphology assessment.
4) Negative for microbial contaminants.
Immortalized Mouse Renal Proximal Tubule Cells originate from mice's renal proximal tubules which play a key role in sodium ion and water reabsorption. The proximal tubule which sits at the renal tubule's entrance functions to reclaim essential substances like sodium, potassium, glucose, and amino acids from the filtrate using both active and passive mechanisms. These cells maintain proper electrolyte balance and body water regulation crucially. Scientists used Lenti-SV40 lentivirus transfection to accomplish cellular immortalization of this cell line. The IMRPTC cell line maintains essential functional properties typical of proximal tubule cells. Research shows that these cells possess and activate sodium/hydrogen exchangers (NHE1), sodium-dependent phosphate transporters (NPT2), and calcium/sodium exchangers (NCX) which play an essential role in kidney sodium absorption. IMRPTC cells engage in transmembrane calcium transport while controlling intracellular calcium levels through PMCA1 and PMCA4 pump expression.
Researchers use IMRPTC cells as models for interstitial fibrosis in diabetic nephropathy while exploring how the YAP signaling pathway affects renal interstitial fibrosis. Moreover, the ability of IMRPTC cells to replicate proximal tubule functions enables researchers to evaluate how drugs affect tubular reabsorption and secretion mechanisms.
D1R Mutants Mistarget to the Non-Raft Domains
Dopamine receptors, notably the D1 receptor (D1R), are pivotal in promoting sodium excretion and blood pressure regulation by inhibiting sodium transporters in renal cells. These receptors localize in lipid rafts/caveolae, specialized microdomains within cell membranes enriched with cholesterol and sphingolipids. Understanding the localization and structural requirements of D1R in these microdomains is essential for elucidating their function in physiological processes such as sodium balance and blood pressure regulation.
Site-directed mutagenesis at ΔD1R-347S did not affect the receptor's targeting to the plasma membrane, as previously reported. Tiu et al. confirmed this by expressing D1R-wild-type (WT) and mutants (ΔD1R-347A, ΔD1R-351A, ΔD1R-218S) in human renal proximal tubule cells (hRPTCs), where they localized in the plasma membrane fraction marked by gamma-glutamyl transferase (GGT) (Fig. 1A). Confocal microscopy also verified their colocalization with the plasma membrane (Fig. 2B). In contrast, the ΔD1R-LL mutant was mainly cytoplasmic. They further assessed the segregation of D1R-WT and mutants into lipid rafts. Expressed in hRPTCs and separated by sucrose gradient ultracentrifugation, D1R-WT was present in both lipid raft and non-raft domains, more prominently in the latter (Fig. 2). Conversely, the D1R mutants were confined to non-raft domains.
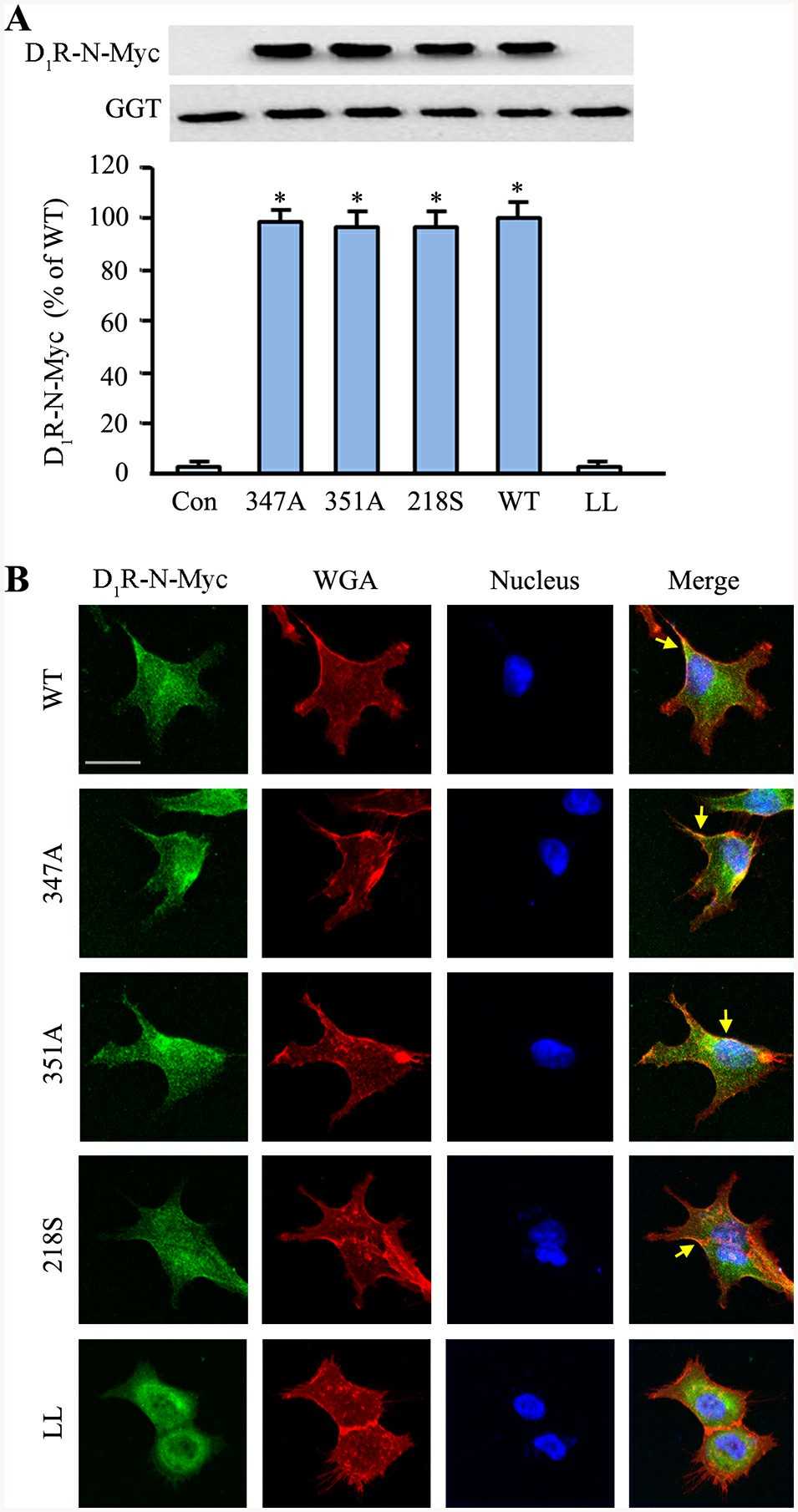 Fig. 1. D1R mutants are targeted to the plasma membrane but not the lipid raft (Tiu AC, Yang J, et al., 2020).
Fig. 1. D1R mutants are targeted to the plasma membrane but not the lipid raft (Tiu AC, Yang J, et al., 2020).
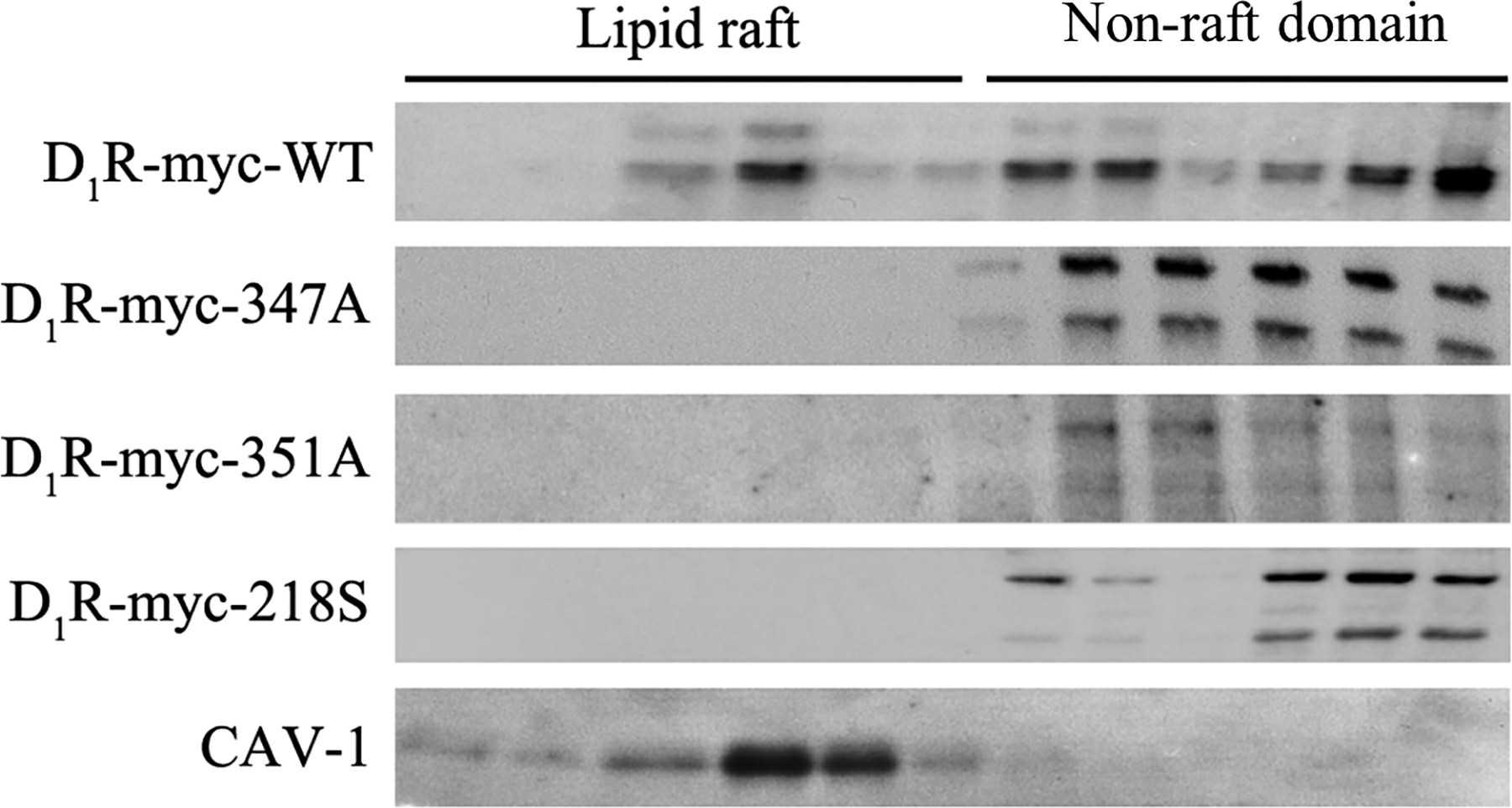 Fig. 2. Lipid raft distribution of D1R-WT and mutants (Tiu AC, Yang J, et al., 2020).
Fig. 2. Lipid raft distribution of D1R-WT and mutants (Tiu AC, Yang J, et al., 2020).
Gastrin, GLP-1, or Gastrin plus GLP-1 Negatively Regulates the Sodium Transporter/Exchanger/Pump in Human Renal Proximal Tubule Cells (hRPTCs)
Excessive sodium intake can lead to hypertension due to ineffective renal sodium excretion, while efficient sodium excretion maintains normal blood pressure. The gastrointestinal tract regulates sodium balance through hormone secretion, including gastrin and GLP-1. Fan's team investigated their effect on SGLT2, Na-K/ATPase, and NHE3 expressions, and NHE3 phosphorylation in immortalized human renal proximal tubule cells (hRPTCs). After 3 hours of incubation, GLP-1 inhibited SGLT2 expression by 45%, while gastrin had no effect but did not alter GLP-1's inhibition (Fig. 3A). Gastrin decreased Na-K/ATPase expression by 34%, with GLP-1 enhancing this effect (Fig. 3B). Both hormones inhibit NHE3 to induce natriuresis. Gastrin and GLP-1 increased the phosphorylation of NHE3 at serine 552 individually and synergistically (Fig. 3D and E), but only their combination increased phosphorylation at serine 605 (Fig. 3E). These results suggest GLP-1 and gastrin have complementary effects on sodium transport.
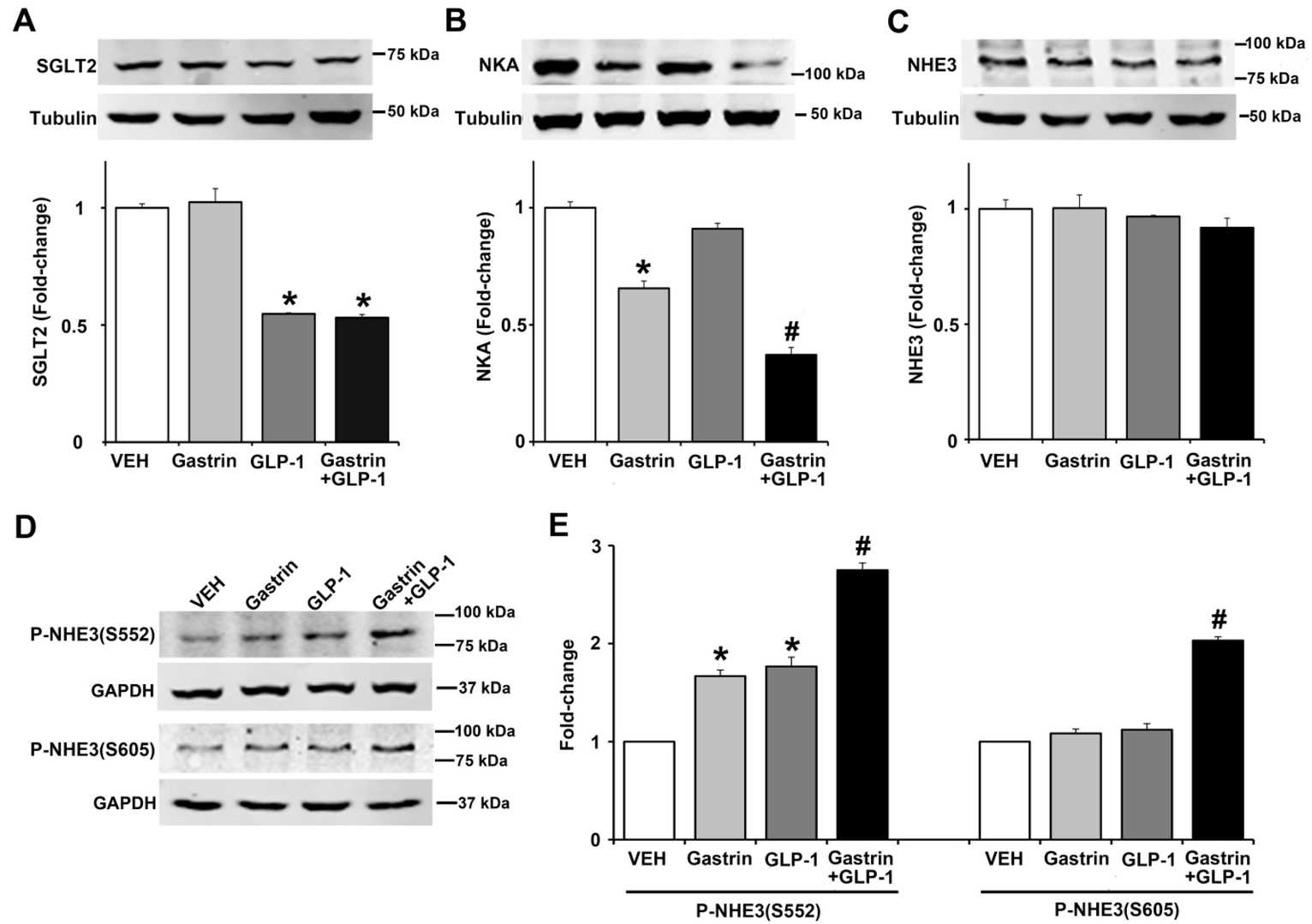 Fig. 3. Effect of gastrin and GLP-1 on sodium transporter/pump/exchanger in human renal proximal tubule cells (hRPTCs). The hRPTCs were treated with gastrin (100 nM), GLP-1 (10 nM), or gastrin plus GLP-1 for 3 h (Fan C, Asico LD, et al., 2021).
Fig. 3. Effect of gastrin and GLP-1 on sodium transporter/pump/exchanger in human renal proximal tubule cells (hRPTCs). The hRPTCs were treated with gastrin (100 nM), GLP-1 (10 nM), or gastrin plus GLP-1 for 3 h (Fan C, Asico LD, et al., 2021).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

