RN46A-B14
Cat.No.: CSC-C9439J
Species: Rattus norvegicus (Rat)
Source: Embryo; Brain
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
"RN46A-B14" is a transformed rat embryo (day 13 myelinated raphe nuclei) cell line that is known for its immortal, serotonergic, and neuronal properties. The cell line was created by isolating a clone after transfected with the gene that encodes for rat brain-derived neurotrophic factor (BDNF) into RN46A cells. RN46A-B14 cells are known to produce and secrete bioactive BDNF in vitro and also have the ability to produce serotonin (5-HT) after partial membrane depolarization.
The RN46A-B14 cell line is commonly used in research for such as the serotonergic pathway, histone modifications, and antidepressant drug effects. Some examples of papers that used the RN46A-B14 cell line include the association of estrogen-mediated 5-HT systems and premenstrual syndrome (PMS) and histone serotonylation modifications and roles in gene expression regulation. Owing to its properties of BDNF and 5-HT secretion, RN46A-B14 cells can be used for neural regeneration and repair research such as in a spinal cord injury model of neurodegenerative diseases.
1,25-dihydroxyvitamin D represses SERT and degradation (MAO-A) gene expression in cultured rat serotonergic neuronal cell lines
To date, low brain serotonin and active vitamin D metabolite (1,25D) have been linked to atypical behavior such as autism and depression. It was previously demonstrated that 1,25D can cause TPH2 expression in rat serotonergic neuronal cells. However, other serotonin pathway components have not been evaluated.
To determine if vitamin D affects the major route of serotonin reuptake and degradation, the influence of 1,25D on the expression of SERT and MAO-A was assessed by quantitative real-time PCR in differentiated serotonergic rat raphe RN46A-B14 cells. Cells in culture were challenged with 1, 10, and 100 nM 1,25D, and mRNA was isolated, reverse transcribed to cDNA and probed for SERT and MAO-A expression. Figure 1a illustrates that SERT is unaffected by 1 and 100 nM 1,25D-treatment, but a concentration of 10 nM 1,25D significantly represses SERT mRNA expression by 59%. Thus, the response of SERT to 1,25D is biphasic, reminiscent of the U-shaped curves generated when plotting circulating 25-hydroxyvitamin D levels versus disease and death in humans. Strikingly, MAO-A displays an identical regulatory pattern of expression when challenged with a range of 1,25D concentrations in differentiated serotonergic rat raphe RN46A-B14 cells (Fig. 1b). MAO-A is similarly unaffected by 1 and 100 nM 1,25D-treatment, but a concentration of 10 nM 1,25D significantly represses MAO-A mRNA expression by 51%. Therefore, two neurally expressed genes exhibit a similar profile of regulation by 1,25D, and the data suggest that certain levels of the vitamin D hormone appear to be capable of suppressing serotonin reuptake and catabolism.
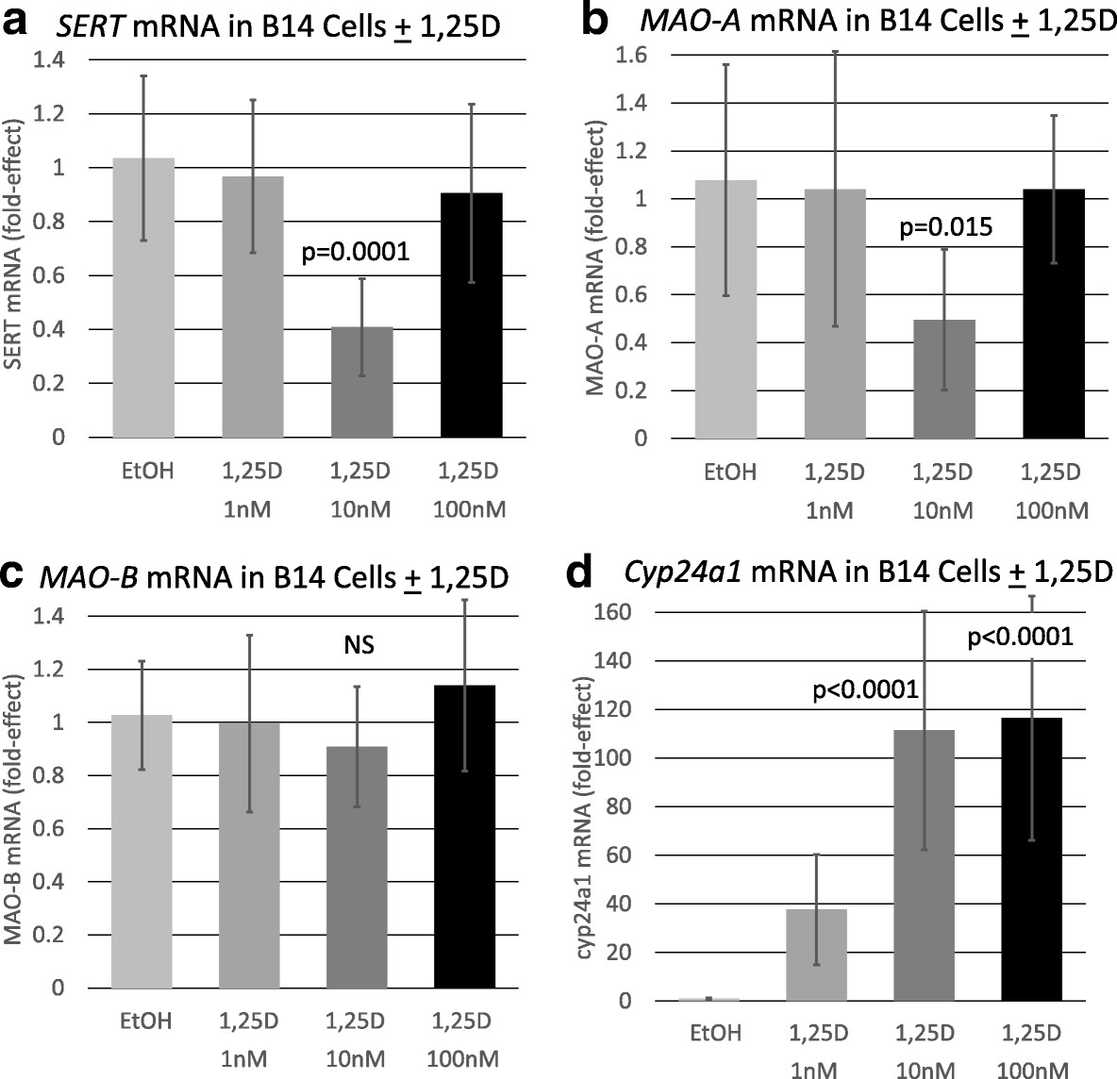 Fig. 1. Effect of 1,25D on selected serotonin degrading pathway and control mRNAs in a serotonergic neuronal cell line (Sabir M S, Haussler M R, et al., 2018).
Fig. 1. Effect of 1,25D on selected serotonin degrading pathway and control mRNAs in a serotonergic neuronal cell line (Sabir M S, Haussler M R, et al., 2018).
Pomegranate Derivative Urolithin a Enhances Vitamin D Receptor Signaling to Amplify Serotonin-Related Gene Induction by 1,25-Dihydroxyvitamin D
The active metabolite of vitamin D (1,25D) up-regulates genes through the vitamin D receptor (VDR). Urolithin A, a pomegranate metabolite, induces mitophagy through AMPK. The two together could alter the expression of neuroendocrine genes.
To assess whether urolithin A also increases 1,25D/VDR driven transcription on native chromatin, Livingston et al. quantified CYP24A1 mRNA using qPCR. 1,25D induced CYP24A1 12.6 fold in HEK293 cells (Fig. 2A). The presence of 10 µM urolithin A increased this induction to 3.3 fold. Urolithin A alone had no effect, consistent with its inability to bind the VDR directly. They next asked whether urolithin A produced similar effects in HEK293 cells (Fig. 2A) and RN46A-B14 cells (Fig. 2B). In RN46A-B14 cells, 1,25D induced cyp24a1 8 fold, which was augmented in the presence of urolithin A to a total induction of 100 fold. They also tested resveratrol as a second nutraceutical compound that has been shown to activate AMPK. Resveratrol augmented 1,25D/VDR-mediated transcription, but to a lesser and non-significant degree. They also observed that resveratrol decreased the potency of urolithin A in the presence of both compounds (Fig. 2B, far right treatment group). Urolithin A is the only nutraceutical they tested that can significantly and potently augment VDR-driven gene induction on native chromatin at a half-maximal concentration of 10 μM.
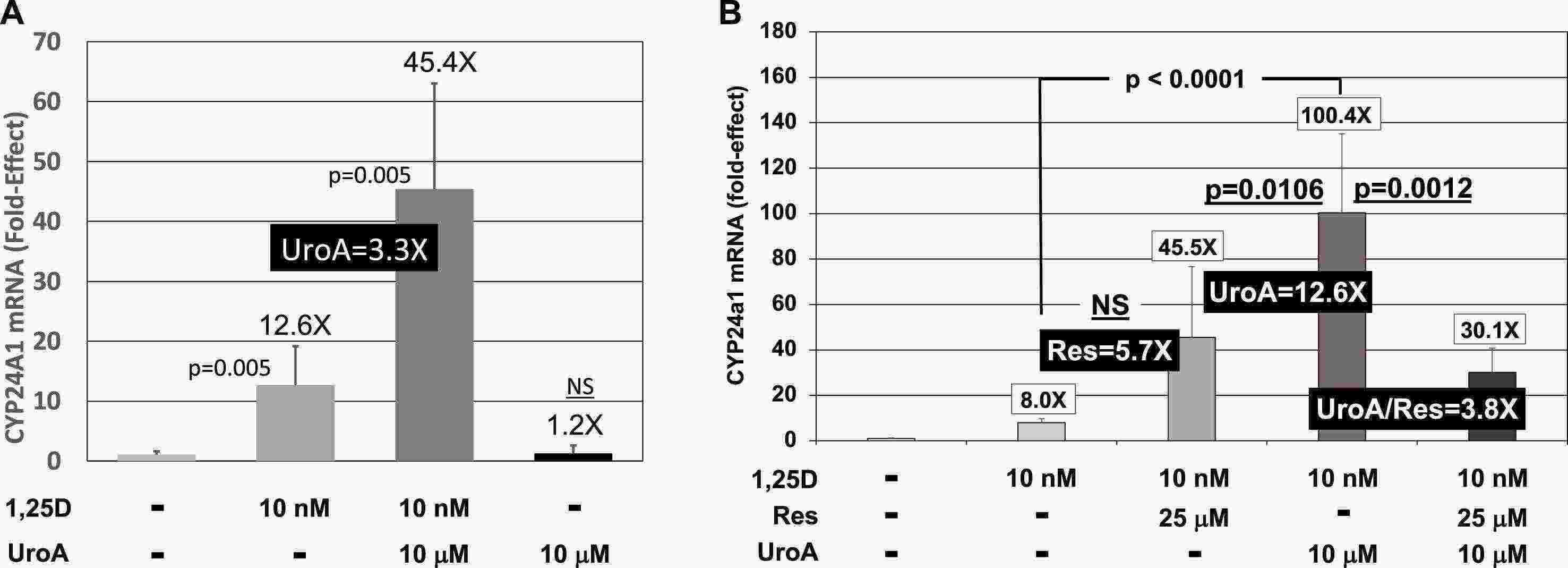 Fig. 2. Effect of 1,25D and UroA and/or Res on CYP24A1 mRNA concentrations in cultured cells (Livingston S, Mallick S, et al., 2020).
Fig. 2. Effect of 1,25D and UroA and/or Res on CYP24A1 mRNA concentrations in cultured cells (Livingston S, Mallick S, et al., 2020).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells