Strain C57BL/6 Mouse Adipose-Derived Mesenchymal Stem Cells with GFP
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The main source of Strain C57BL/6 Mouse Adipose-Derived Mesenchymal Stem Cells with GFP comes from subcutaneous adipose tissues of the inguinal and groin areas. Researchers can extract stem cells from these adipose tissues by applying mechanical separation along with enzymatic digestion techniques. The cells that have been isolated demonstrate high multipotency capabilities by differentiating into several cell types including osteoblasts, adipocytes, chondrocytes, myocytes, and neurons. Research studies in controlled lab settings show these cells can transform into distinct cell types when subjected to specific induction protocols which scientists confirmed through immunocytochemistry techniques and RT-PCR analysis.
These cells possess distinctive benefits beyond their use in cell differentiation research as well as in tissue engineering and regenerative medicine. GFP marks the cells which enables scientists to monitor their movement and localization processes through real-time fluorescence microscopy. Researchers can use this feature to examine cell activity in living organisms while investigating their contributions to tissue healing and regeneration.
Single-Cell Transcriptomics of mADSCs-Treated and Untreated Pulmonary Resident Cells
Idiopathic pulmonary fibrosis (IPF) is a severe lung disease with few effective treatments. ADSCs have demonstrated potential benefits for IPF, but the underlying mechanisms are unknown. In this study, bleomycin (BLM) was used to induce pulmonary fibrosis in mice, after which GFP-labeled mouse adipose-derived mesenchymal stem cells (mADSCs) were intratracheally implanted. The mADSCs were retrieved and analyzed via single-cell RNA sequencing to investigate their impact on the macrophage profile and overall lung microenvironment.
Firstly, Rahman et al. analyzed single-cell profiles of lung parenchyma cells in BLM + PBS and BLM + mADSCs groups (Fig. 1b), containing 8181 and 6405 cells respectively. Both groups revealed 27 cell clusters, and 12 cell types were identified based on markers, without cell cycle impact (Fig. 1b). The lung cell types included pneumocytes, bronchiolar exocrine cells, natural killer cells, fibroblasts/epithelial cells, myofibroblasts, dendritic cells, endothelial cells, T cells, B cells, monocytes/macrophages, and granulocytes (Fig. 1b, c). Key findings showed monocytes/macrophages (48.78%) in the BLM + mADSCs group, and granulocytes (44.87%) in the BLM + PBS group as prevalent immune cells (Fig. 2d). The primary non-immune cells in BLM + mADSCs were fibroblasts, endothelial cells, and myofibroblasts, occupying 7.45, 6.83, and 3.80% respectively. Monocytes/macrophages were also predominant in both groups BLM + mADSCs (48.78%) and BLM + PBS (27.28%) (Fig. 2a), split into 9 subclasses based on gene expression (Fig. 2b). Mo/Ma-1, marked by MPG, COL1a1, and SPARC, was the largest subgroup (48.71%), whereas macrophage-4 expressing GPNMB, PF4, and APOE was the second largest in BLM + mADSCs (20.42%). Alveolar macrophage-21 and Mo/Ma-24 were negligible in BLM + PBS (0 and 0.07%) (Fig. 2b). Further analysis on Mo/Ma-1 and macrophage-4 subclasses showed they impact ECM regulation and related pathways with genes like COL1a1, COL1a2, and SPARC highly expressed (Fig. 2c, d).
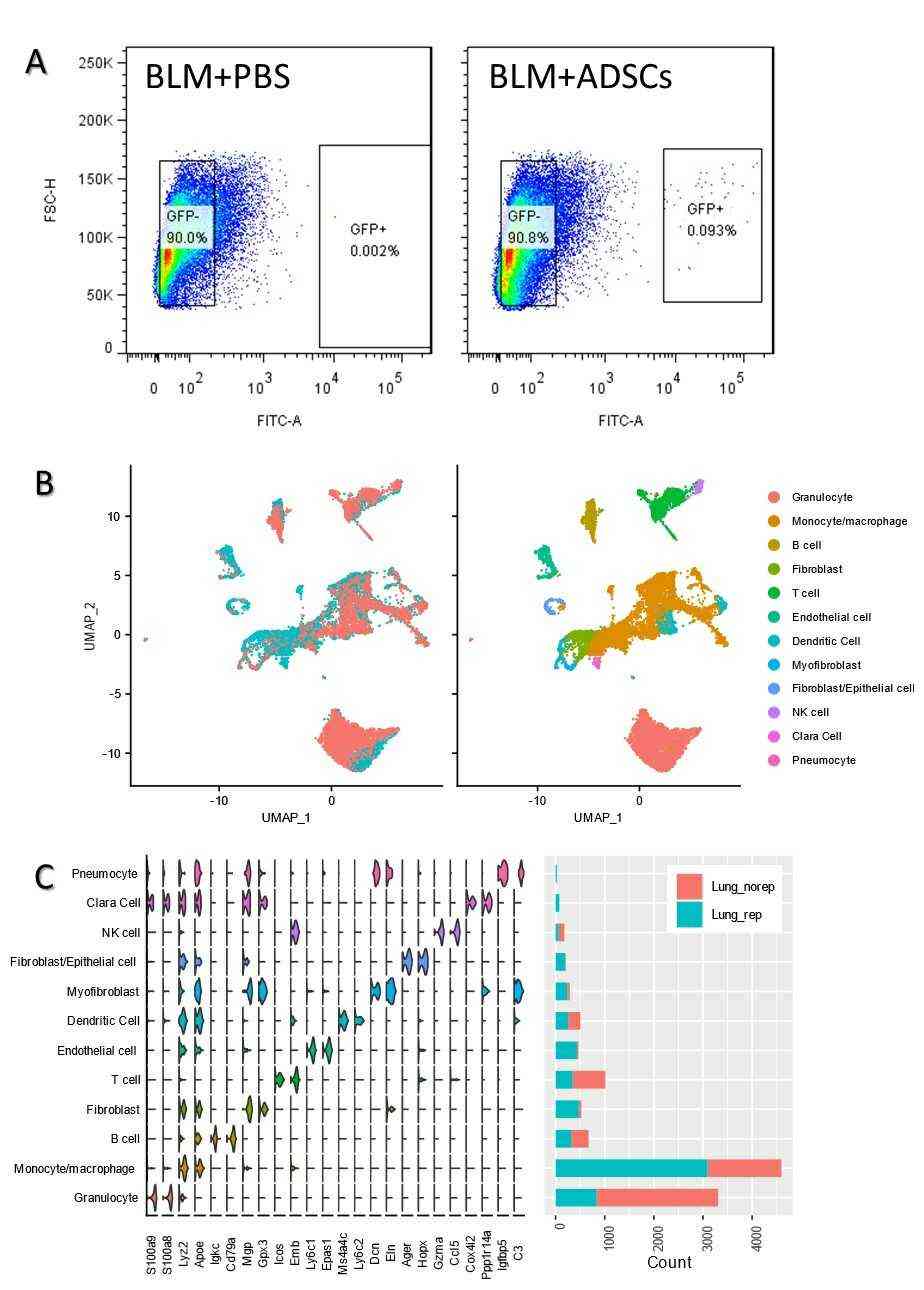 Fig. 1. Injection of mADSCs through the intratracheal caused increase of monocyte/macrophage population (Rahman M, Wang Z Y, et al., 2021).
Fig. 1. Injection of mADSCs through the intratracheal caused increase of monocyte/macrophage population (Rahman M, Wang Z Y, et al., 2021).
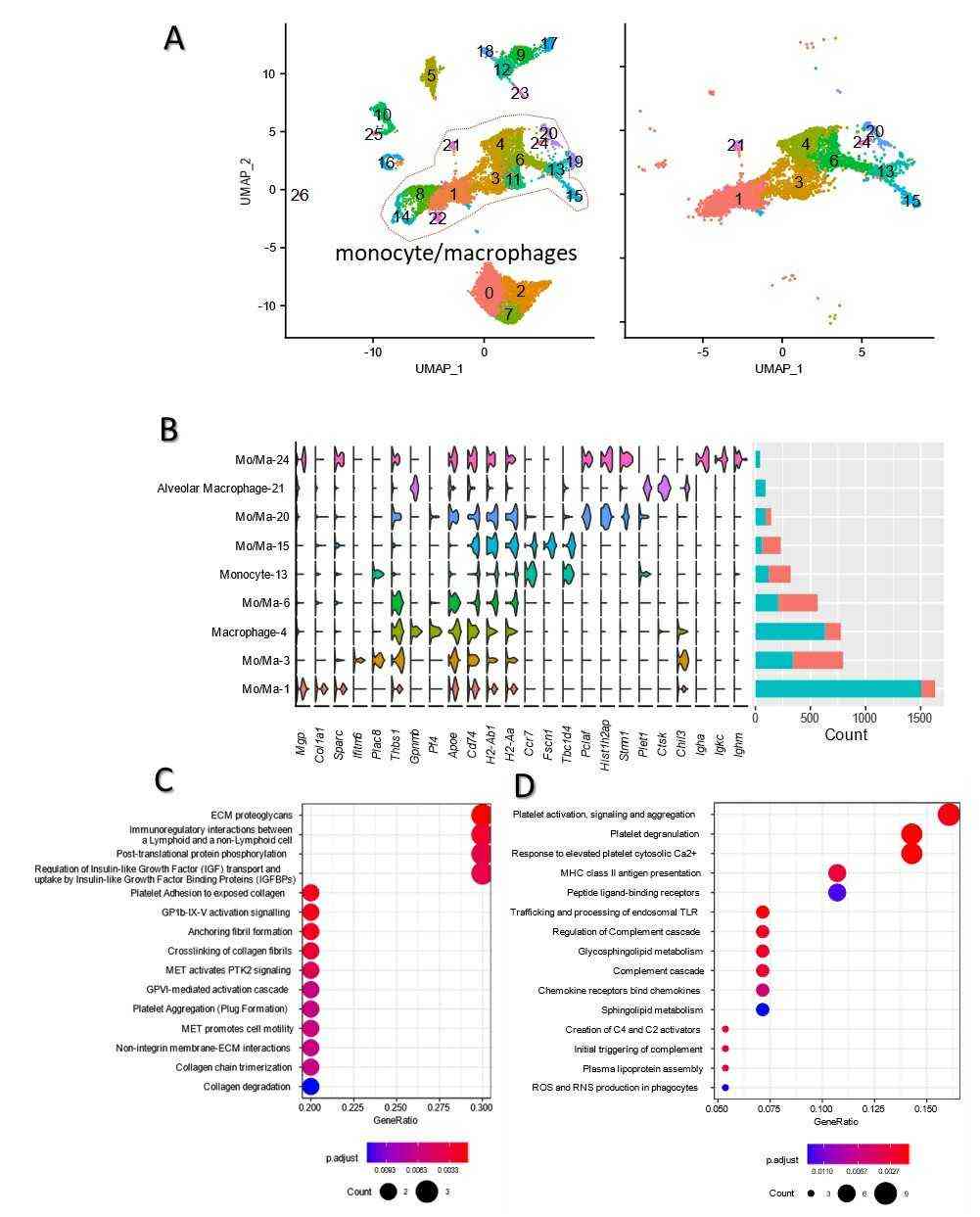 Fig. 2. Go enrichment and KEGG analysis of monocyte/macrophage (a) UMAP distribution of 9 monocyte/macrophage clusters in both groups (Rahman M, Wang Z Y, et al., 2021).
Fig. 2. Go enrichment and KEGG analysis of monocyte/macrophage (a) UMAP distribution of 9 monocyte/macrophage clusters in both groups (Rahman M, Wang Z Y, et al., 2021).
Adipose-Derived Mesenchymal Stem Cell Seeded Atelocollagen Scaffolds for Cardiac Tissue Engineering
Stem cell transplantation presents a possible treatment option for myocardial infarction through the engineering of heart tissue with diverse biological materials. Research indicates that Atelocollagen displays promising scaffold properties because of its ability to function well with biological tissues. Adipose-derived mesenchymal stem cell (ADMSCs) can be readily extracted from their source and show promise in tissue engineering applications.
Li's team explored the biocompatibility of mouse ADMSCs-GFP with Atelocollagen scaffolds and their ability to differentiate into cardiomyocytes for myocardial tissue engineering. Proliferation of ADMSCs on the collagen scaffolds of Atelocollagen was evaluated by measuring the fluorescence of GFP labeled ADMSCs. The ADMSCs grew rapidly on the collagen scaffolds of Atelocollagen, and the cells can grow into the mesh of the collagen scaffolds. On the 7th day, about 50% of the mesh of the scaffolds were filled with GFP-labeled ADMSCs (Fig. 3). ADMSCs were cultured on 3D Atelocollagen scaffolds. After 3 weeks of 5-aza induction, cTnT immunofluorescence was positive (Fig. 4).
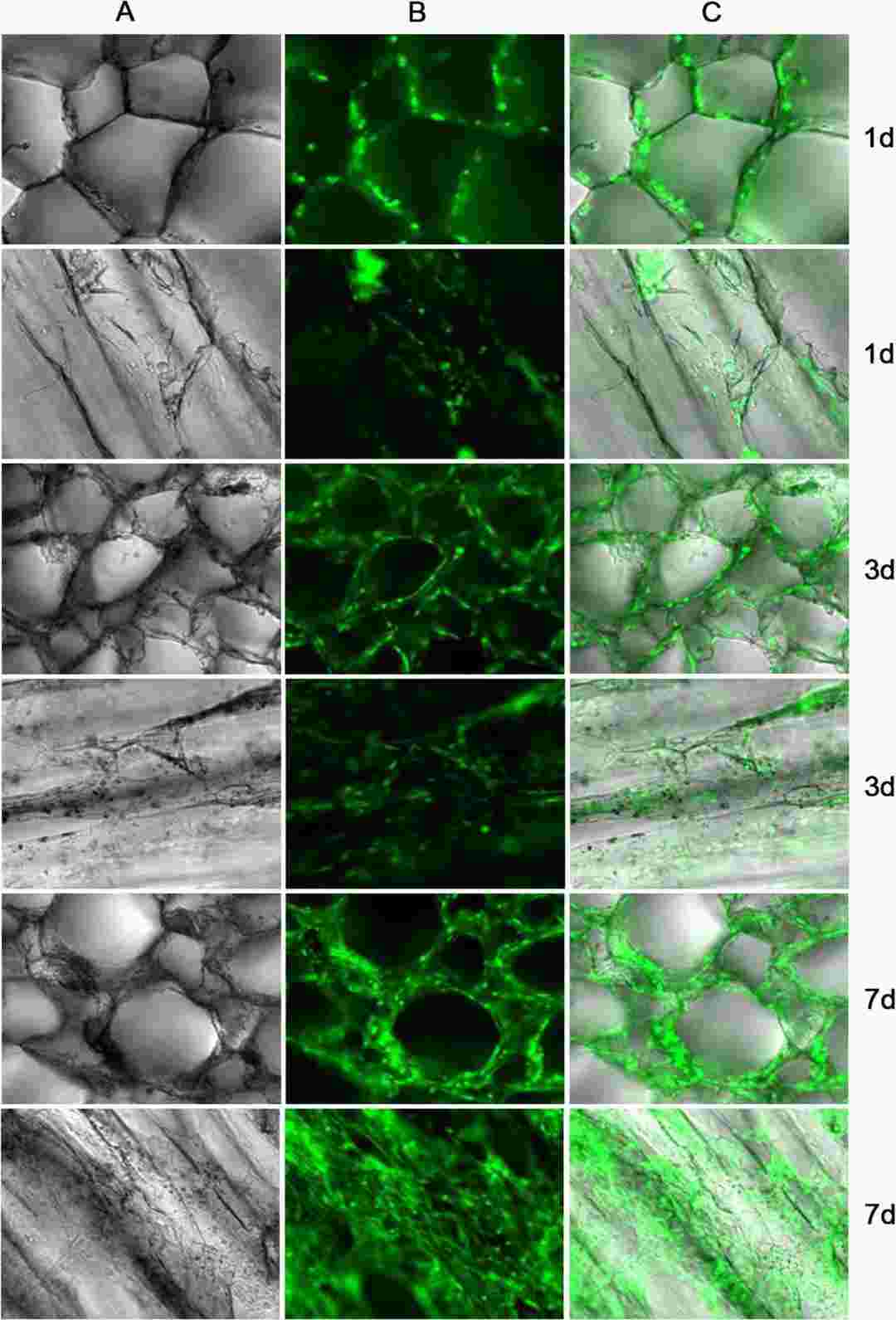 Fig. 3. The image of ADMSCs compatible with the Atelocollagen scaffolds (Li Q, Li MM, et al., 2020).
Fig. 3. The image of ADMSCs compatible with the Atelocollagen scaffolds (Li Q, Li MM, et al., 2020).
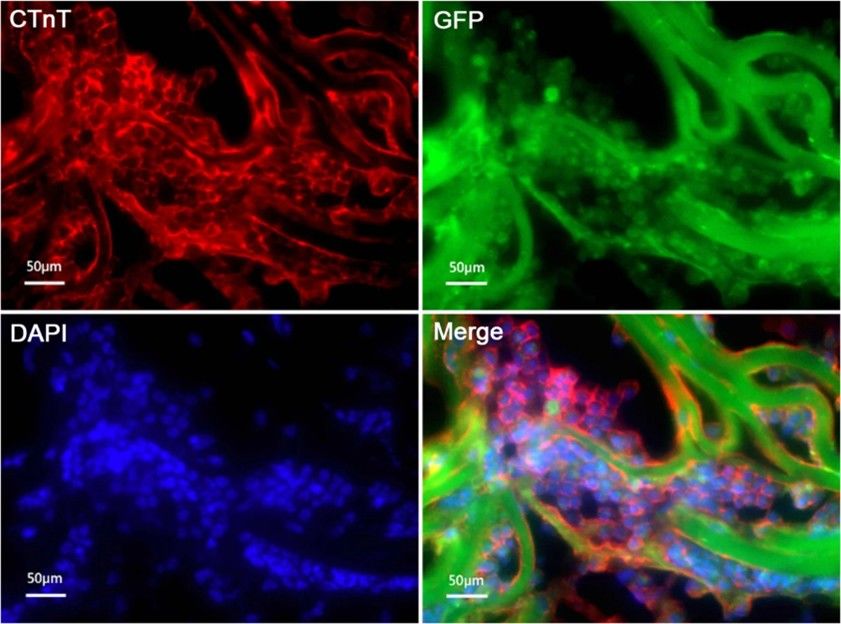 Fig. 4. cTnT expression of ADMSCs induced 3 weeks with 5-aza on 3D scaffolds by immunofluorescence staining (Li Q, Li MM, et al., 2020).
Fig. 4. cTnT expression of ADMSCs induced 3 weeks with 5-aza on 3D scaffolds by immunofluorescence staining (Li Q, Li MM, et al., 2020).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells