
Immortalized Mouse Dermal Fibroblasts-SV40
Cat.No.: CSC-I2190Z
Species: mouse
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
free from contaminations (bacteria incl. mycoplasma, fungi, HIV, HAV, HBV, HCV, Parvo-B19) and cross-contaminations
Note: Never can cells be kept at -20°C.
The Immortalized Mouse Dermal Fibroblasts-SV40 cell line has various applications in different research fields and disciplines. Firstly, it can be used to study skin physiology and pathology, which can help scientists to explore the physiological functions of the skin, the metabolism of the extracellular matrix, and the pathogenesis of skin diseases such as skin fibrosis and inflammation. Secondly, it can also be used for drug screening and toxicity testing to discover therapeutic drugs. Scientists can evaluate the effects of drugs on cell proliferation, extracellular matrix synthesis, and cell survival by using them, and thus screen out drugs with good efficacy and safety. Thirdly, it can be used in tissue engineering and regenerative medicine. As seed cells, it can be mixed with biomaterials to construct skin tissue engineering scaffolds, which can provide a powerful research tool for skin tissue repair and regeneration.
VPA Induced Primary Porcine Hepatocytes to Reprogram into Hepatic Progenitor Cells In Vitro
Hepatocyte transplantation is considered a feasible and cost-effective alternative to liver transplantation for end-stage liver disease. Human hepatocytes are limited and porcine hepatocytes are a potential alternative. However, the long-term in vitro proliferation of porcine hepatocytes is restricted.
Li's team developed a method to stimulate the in vitro proliferation of primary porcine hepatocytes using Valproic acid (VPA) (Fig. 1a). The growth-stimulating medium was named VPA-HCM, and it contained 0.5 mM VPA added to HCM. Primary pig hepatocytes (PPH) were rapidly proliferated in VPA-HCM (Fig. 1c) and the nucleus-to-cytoplasm ratio was increased at seven days compared to that of primary pig liver cells (Fig. 1b). Immunofluorescence staining showed that compared with freshly isolated pig liver cells, cells cultured in VPA-HCM for seven days expressed significantly higher cell cycle marker (MKI67 and PCNA) and HPC marker (KRT19 and SOX9) and hepatocyte function marker (HNF1A, HNF4A and Albumin) was decreased (Fig. 1d, e). The cell morphology changed from that of a typical hepatocyte to that of a progenitor cell after 7 days of culture in VPA-HCM, and they were called Valproic acid-induced Hepatic Progenitor Cells (VPA-iHPCs). VPA-iHPCs (D7) upregulated HPC and cell cycle genes and downregulated mature hepatocyte function genes in the qPCR assay (Fig. 1f). In summary, VPA-induced the differentiation of primary porcine hepatocytes into high-proliferation HPCs.
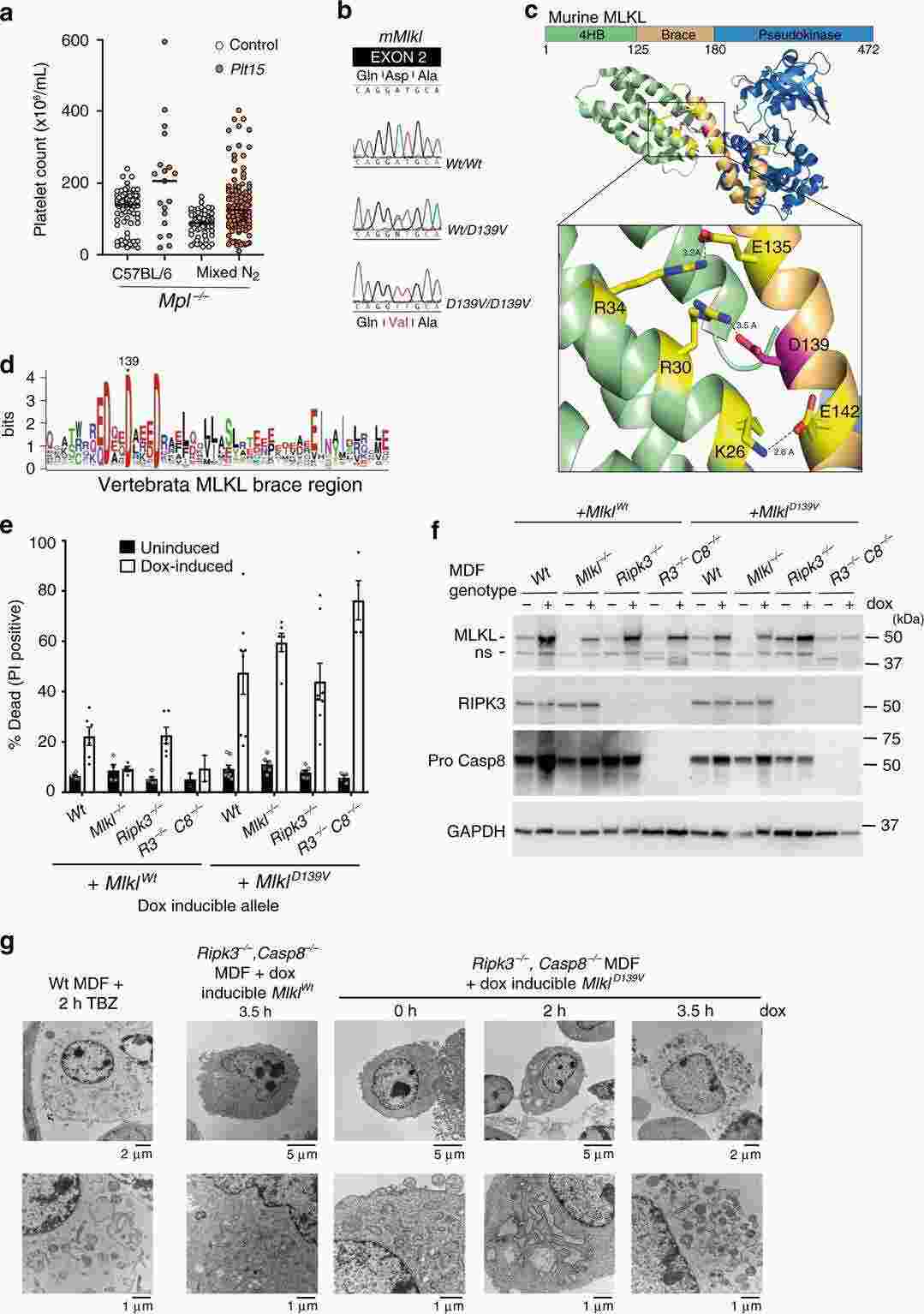 Fig. 1. VPA induced primary porcine hepatocytes to reprogram into Hepatic Progenitor Cells in vitro (Li G H, Zeng M, et al., 2024).
Fig. 1. VPA induced primary porcine hepatocytes to reprogram into Hepatic Progenitor Cells in vitro (Li G H, Zeng M, et al., 2024).
Traversal of Porcine Hepatocytes by Plasmodium falciparum Sporozoites
Plasmodium falciparum (Pf) causes severe malaria by infecting human hepatocytes. The molecular mechanisms of host specificity and maturation in non-human hepatocytes are poorly understood. Here, van der Boor et al. used fresh porcine hepatocytes to study Pf sporozoite invasion and development.
Different seeding densities were tested, with viability assessed using brightfield microscopy and MTT from the day of invasion to day five (Fig. 2A and B). Metabolic activity, measured by MTT, increased up to 72 hours post-invasion for all densities and then stabilized (Fig. 2B). Beyond day seven, cell viability decreased, showing clumping under microscopy. An optimal density of 62,500 was selected, as higher densities led to cell clumping rather than monolayer formation, aligning with densities used for primary human hepatocytes. Both human and porcine hepatocytes were infected with NF135.C10 sporozoites two days post-plating. After a three-hour traversal assay, significant FITC dextran-positive cells indicated NF135.C10 sporozoites could traverse porcine hepatocytes, though less efficiently than human ones. Traversal was less in porcine than in human hepatocytes (Fig. 2C and D).
 Fig. 2. (A-B) Microscopic images and viability of freshly seeded porcine hepatocytes. (C-D) Traversal of NF135.C10 sporozoites in fresh human and porcine hepatocytes three hours post-invasion of three biological donors (van der Boor SC, van Gemert GJ, et al., 2022).
Fig. 2. (A-B) Microscopic images and viability of freshly seeded porcine hepatocytes. (C-D) Traversal of NF135.C10 sporozoites in fresh human and porcine hepatocytes three hours post-invasion of three biological donors (van der Boor SC, van Gemert GJ, et al., 2022).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells
