
Immortalized Duck Embryo Fibroblasts
Cat.No.: CSC-I2063Z
Species: duck
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
free from contaminations (bacteria incl. mycoplasma, fungi, HIV, HAV, HBV, HCV, Parvo-B19) and cross-contaminations
Note: Never can cells be kept at -20°C.
Immortalized Duck Embryo Fibroblasts (IDEF) are fibroblasts isolated from 11-14 days old duck embryos. Fibroblasts are one type of cell that has differentiated from mesenchymal cells during the embryonic development stage. They are commonly found in the connective tissues of the embryo, such as the mesenchymal tissues in the skin, muscles, and bones. Fibroblasts deliver necessary support and nutrition during embryonic development stages. They also provide a microenvironment for the growth and differentiation of other cells in the embryo.
Duck embryo fibroblasts are an excellent cell model for the study of waterfowl viruses. Many waterfowl viruses, such as duck plague virus, duck hepatitis virus, and duck Tembusu virus, can proliferate well in duck embryo fibroblasts. By culturing viruses in duck embryo fibroblasts, researchers can isolate, identify, titrate the virus, and conduct studies on virus replication mechanisms. In addition, using duck embryo fibroblasts can study the interactions between viruses and host cells, including virus invasion, replication, assembly, release, and the immune response of host cells to viral infection.
Induction of Adipogenesis in CCL-141 Cells by Different Culture Components
Ducks are important for poultry meat production, with fats in their meat influencing taste and quality. Adipogenesis, regulated by multiple genes, is crucial for breeding desirable fat traits. Previous studies used primary cells, which are limited for gene editing. This study used chicken serum, fatty acids, insulin, and all-trans retinoic acid to induce adipogenesis in immortalized duck embryo fibroblasts CCL-141 cells, confirmed by Oil Red O staining and gene expression analysis.
Findings show duck embryo fibroblasts have enhanced adipogenic potential under chicken serum, oleic acid, linoleic acid, insulin, atRA, etc. Compared to the fetal bovine serum control, lipid deposition increased in groups b, c, and d (Fig. 1A), with significant differences among groups (Fig. 1B, p < 0.05). Group d (added atRA) had the highest deposition, indicating atRA's strong pro-adipogenic effect. Group b (chicken serum) showed few lipid droplets, while group a (fetal bovine serum) showed almost none, suggesting chicken serum contains pro-adipogenic components. Adding fatty acids and insulin (group c) further increased deposition compared to group b, indicating their enhancing effect. Adipogenic gene expression was higher at 72 h than at 48 h (Fig. 2), better reflecting differentiation status. Therefore, 72 h samples were used for lipid droplet assays.
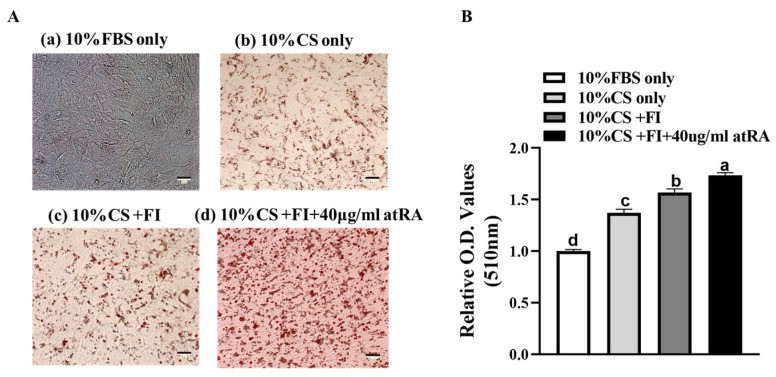 Fig. 1. Inducing effects of different culture medium components on immortalized duck embryo fibroblasts CCL-141 cells (Sun D D, Li X Q, et al., 2024).
Fig. 1. Inducing effects of different culture medium components on immortalized duck embryo fibroblasts CCL-141 cells (Sun D D, Li X Q, et al., 2024).
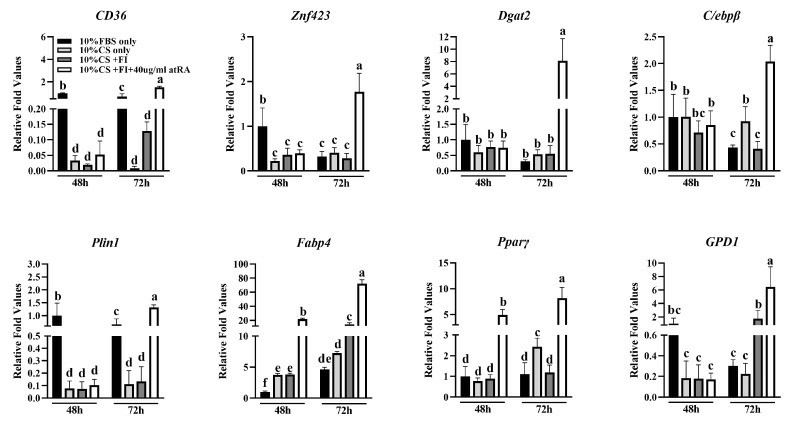 Fig. 2. Differential expression of marker genes for adipogenesis in differentiating groups induced by culture medium containing different components for 48 h and 72 h (Sun D D, Li X Q, et al., 2024).
Fig. 2. Differential expression of marker genes for adipogenesis in differentiating groups induced by culture medium containing different components for 48 h and 72 h (Sun D D, Li X Q, et al., 2024).
Experimental Pathogenesis of Aquatic Bird Bornavirus 1 in Pekin Ducks
Aquatic bird bornavirus 1 (ABBV-1) is a neurotropic virus causing persistent infection in wild waterfowl, associated with neurological signs and inflammation. Previous studies showed ABBV-1 infection in Muscovy ducks and chickens, with minimal clinical signs but histologic lesions.
The ABBV-1 strain for this experiment was initially isolated from the brain of a naturally infected Canada goose, and propagated in immortalized duck embryo fibroblasts. Then, Pekin ducks were inoculated with ABBV-1 via intracranial, intramuscular, and choanal routes. Virus distribution, lesions, and clinical signs were assessed at 1, 12, and 21 weeks post-infection. Perivascular cuffs, made of lymphocyte-like mononuclear cells, were randomly distributed in the cerebrum, cerebellum, brainstem, and spinal cord (Fig. 3a–c). These cuffs were mainly in IC and IM ducks at 12 and 21 wpi, with only one CH duck (#461) showing them at 21 wpi. All inflamed birds were positive for ABBV-1 RNA in the CNS. Inflammation peaked at 12 wpi, with most IC (7/9; 77.8%) and IM (6/8; 75.0%) ducks affected, dropping sharply by 21 wpi, with perivascular cuffs only in 1 IC and 5 IM ducks. Lesion severity also peaked at 12 wpi and dropped by 21 wpi (Fig. 4). In the IC group, cerebrum inflammation score at 12 wpi was significantly higher than in the brainstem and the cerebrum at 21 wpi (Fig. 4a). In the IM group, inflammation severity decreased from 12 to 21 wpi, though not significantly (Fig. 4b).
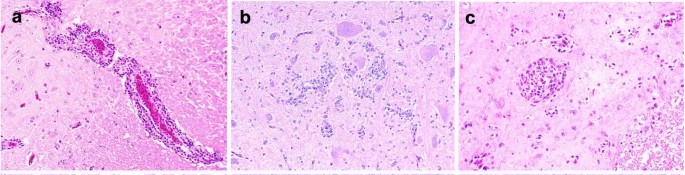 Fig. 3. Microscopic lesions observed in Pekin ducks experimentally infected with aquatic bird bornavirus 1 (ABBV-1) through the intracranial (IC), intramuscular (IM), and choanal (CH) routes at 12 weeks postinfection (Ampuero F, Leacy A, et al., 2023).
Fig. 3. Microscopic lesions observed in Pekin ducks experimentally infected with aquatic bird bornavirus 1 (ABBV-1) through the intracranial (IC), intramuscular (IM), and choanal (CH) routes at 12 weeks postinfection (Ampuero F, Leacy A, et al., 2023).
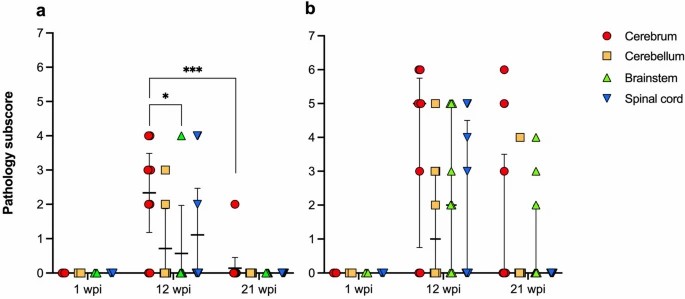 Fig. 4. Pathology subscores for inflammation in different areas of the central nervous system of Pekin ducks experimentally inoculated with aquatic bird bornavirus-1 (ABBV-1) intracranially (a) and intramuscularly (b) (Ampuero F, Leacy A, et al., 2023).
Fig. 4. Pathology subscores for inflammation in different areas of the central nervous system of Pekin ducks experimentally inoculated with aquatic bird bornavirus-1 (ABBV-1) intracranially (a) and intramuscularly (b) (Ampuero F, Leacy A, et al., 2023).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

