Strain C57BL/6 Mouse Mesenchymal Stem Cells with RFP
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Strain C57BL/6 mouse mesenchymal stem cells (MSCs) with RFP are derived from C57BL/6 mice, which represent a standard inbred strain utilized for genetic research because of their consistent genetic characteristics and user-friendly handling properties. The cell line expressing RFP allows scientists to track cell distribution and movement along with differentiation processes in living organisms which renders it ideal for live imaging research.
The cells exhibit strong proliferation rates in laboratory conditions and demonstrate stable growth when cultured in DMEM medium with 10% fetal bovine serum and 1% penicillin-streptomycin. The cells maintain their multipotent differentiation capability during passaging and can transfer into osteoblasts, adipocytes, or chondrocytes when exposed to appropriate environmental conditions. When transplanted in vivo C57BL/6 mouse MSCs show strong homing abilities by migrating to numerous tissues including the liver, spleen and bone marrow.
Enhanced In Vivo Delivery of Stem Cells using Microporous Annealed Particle Scaffolds
Stem cell therapies, especially using mesenchymal stem cells (MSCs), hold promise for disease treatment by repairing tissue and modulating the immune system. Yet, delivery challenges such as insufficient cell retention and environment integration remain. Koh et al. employs microporous annealed particle (MAP) scaffolds to address these issues.
Results showed that cells spread and migrated more rapidly in MAP scaffolds, with increased void volume leading to higher proliferation rates. RFP-MSCs in MAP scaffolds showed a 17-fold expansion over two weeks, compared to a 2.3-fold increase in nonporous gels (Fig. 1B). This enhanced proliferation was confirmed by lower cell-free regions in MAP scaffolds (Fig. 1C). Based on these in vitro results and the robust cell ingrowth in acellular MAP scaffolds in vivo, they hypothesized that MAP scaffolds would improve MSC retention in vivo compared to PBS or nonporous scaffolds. RFP-MSCs were injected subcutaneously into immunocompetent mice, and fluorescence intensity was measured over two weeks (Fig. 1D, E). At one week, RFP intensity was highest in MAP scaffolds (Fig. 1F), with significantly larger cell areas (Fig. 1G). The half-life of MSCs was 6.13 days for MAP scaffolds, 2.35 days for nonporous gels, and 1.91 days for PBS. These results indicate that microporous scaffolds enhance cell proliferation and survival in vivo, likely due to improved transport and cell connectivity.
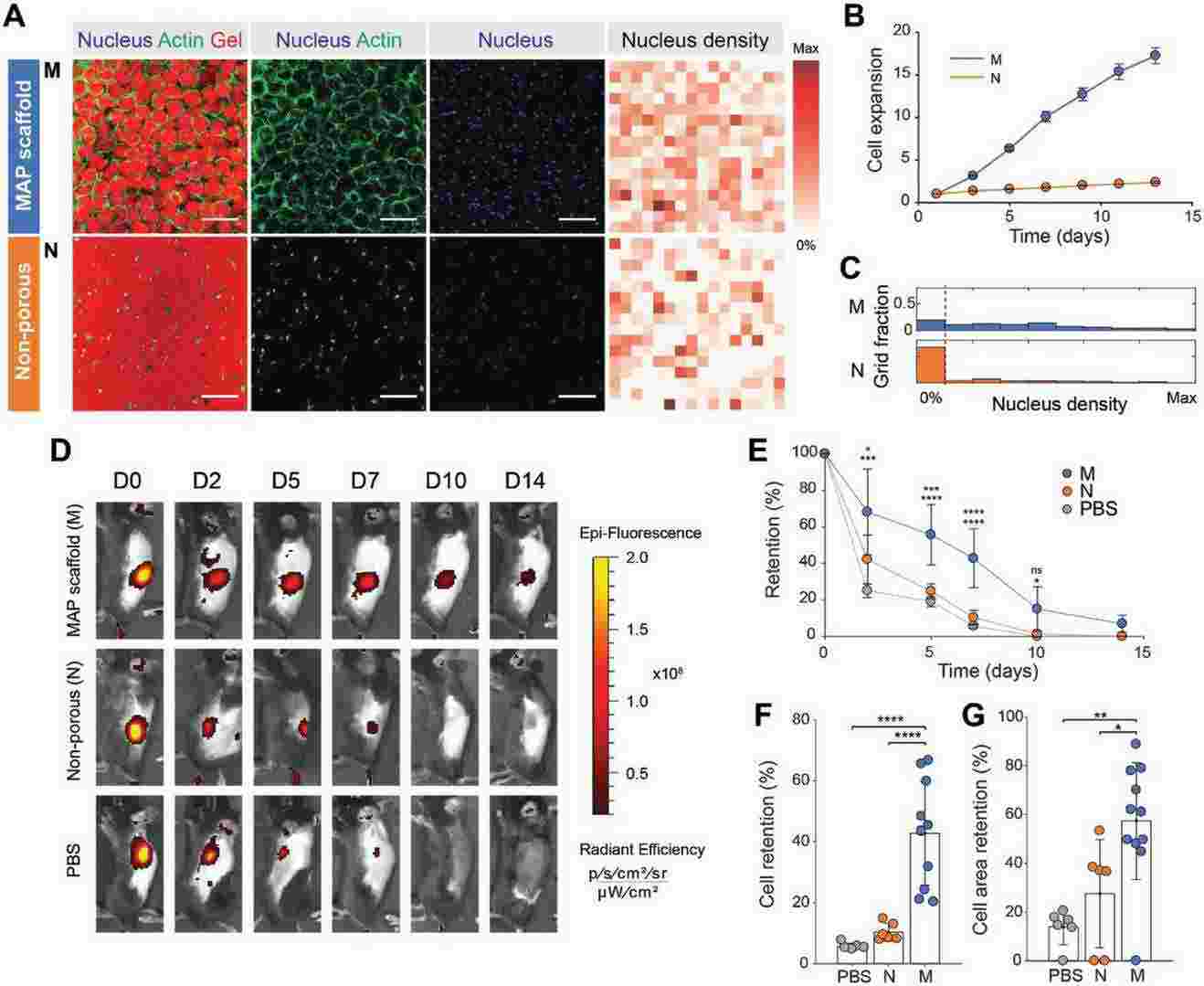 Fig. 1. Controlled microporosity generated by MAP scaffolds facilitates the highest proliferation in vitro and retention in vivo (Koh J, Griffin D R, et al., 2019).
Fig. 1. Controlled microporosity generated by MAP scaffolds facilitates the highest proliferation in vitro and retention in vivo (Koh J, Griffin D R, et al., 2019).
The Presence of CXCR4 is Essential for MSC Homing to the Fracture Site
The development of delayed bone union progresses into non-union which creates severe dysfunction that complicates treatment approaches. BMSCs show promise for enhancing fracture healing but scientists have yet to determine the best time to administer injections. Wang's team investigates the optimal BMSC injection times for enhancing fracture healing in a murine model by studying how different time points affect BMSC migration and bone repair processes.
To assess MSC homing at the femur fracture site, they injected RFP-transfected BMSCs (RFP-BMSCs) into mice on days 1, 7, and 14 post-fracture. In fluorescence imaging analysis, following a day-1 injection, RFP signal intensity was greater on days 7 and 14 when RFP-BMSCs were injected in the absence of AMD3100, although signal intensity was similar in both groups on day 28. A similar pattern of RFP signal intensity was seen after BMSC injection on day 14, although the overall signal intensity was higher following day 7 injection between with and without AMD3100 (Fig. 2). At day 42 post-fracture, signal intensity did not differ between BMSC injections on days 1, 7, and 14. Therefore, a higher RFP signal intensity was maintained following RFP-BMSC injection on day 7 post-fracture.
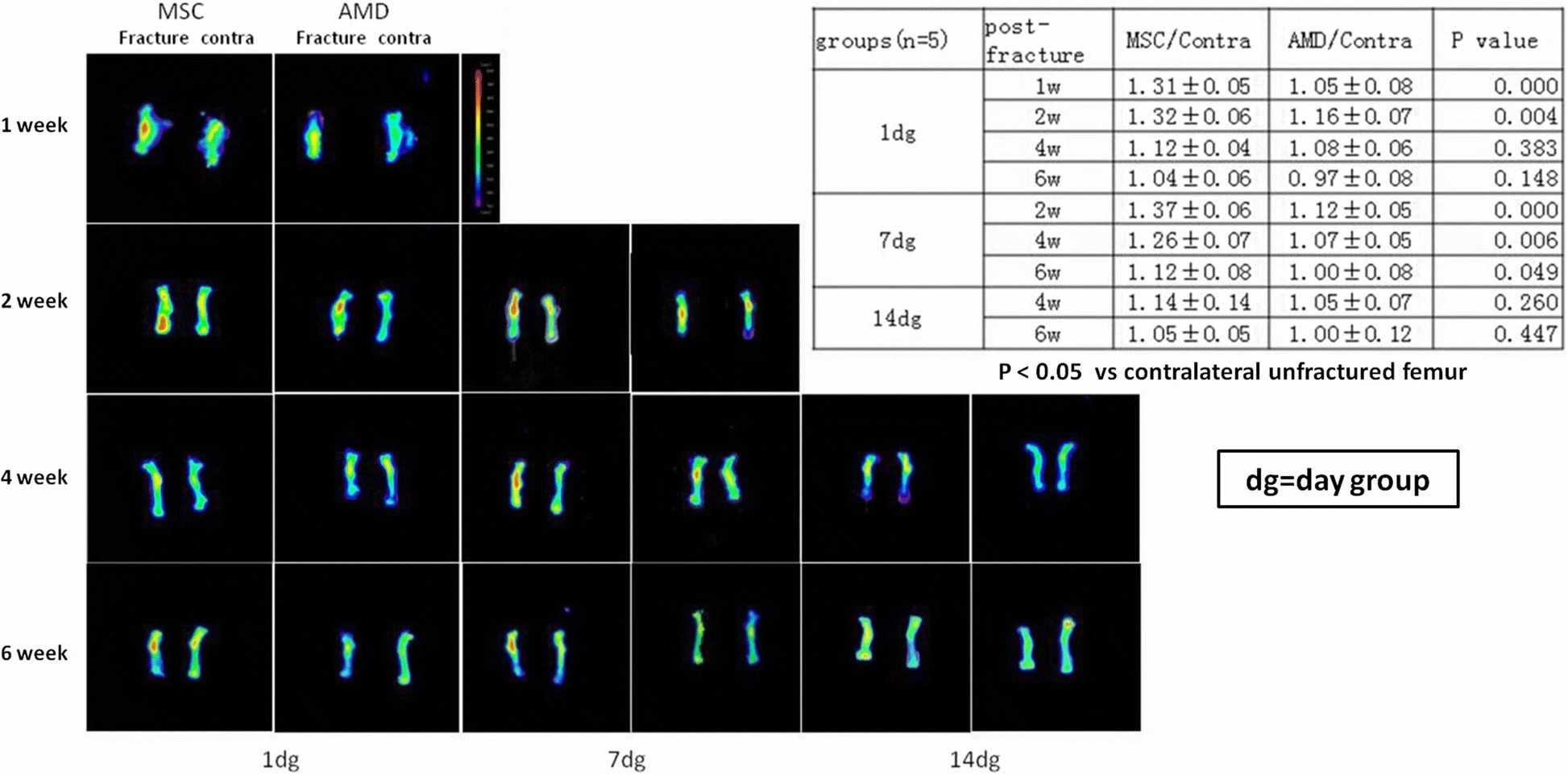 Fig. 2. Representative photographs of fluorescence imaging and semiquantitative analysis (Wang X, Wang C, et al., 2018).
Fig. 2. Representative photographs of fluorescence imaging and semiquantitative analysis (Wang X, Wang C, et al., 2018).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells