Human Osteoclast Precursor Cells
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Osteoclast precursors yield =70 percent conversion to mature and functional Osteoclasts when differentiated using Osteoclast Precusors Medium Kit.
Human Osteoclast precursor cells (OCPs) are precursors of osteoclasts that are derived from hematopoietic stem cells in the bone marrow and belong to the monocyte lineage. OCPs, as well as other myeloid progenitor cells like macrophages and dendritic cells, have a common precursor but are phenotypically and morphologically distinct. OCPs in early stages of development are mononuclear and polygonal. After being induced by M-CSF and RANKL to fuse into multinucleated giant cells, the mature OCPs have a "ruffled border" and "clear zone" to support bone-resorptive activity. OCPs express markers such as tartrate-resistant acid phosphatase (TRAP), calcitonin receptor (CTR), and vitronectin receptor (VNR), which persist after differentiation into osteoclasts.
The main function of osteoclast precursor cells is to differentiate into osteoclasts to promote bone resorption and remodeling. OCPs help to dissolve the bone matrix by secreting acidic substances and proteases (TRAP, Cathepsin K), and to maintain the calcium and phosphorus balance in the body. OCPs are activated in the pathogenesis of osteoporosis, tumor-induced bone metastasis, and rheumatoid arthritis, and thus result in bone loss. Hence, the investigation of the mechanisms underlying the differentiation and activation of osteoclast precursor cells can lead to the discovery of drugs that inhibit bone resorption.
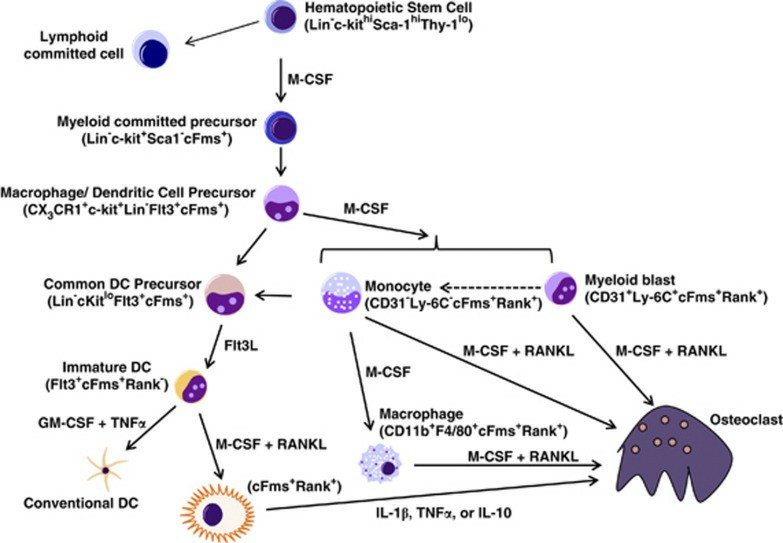 Fig. 1. Osteoclast precursor development and differentiation (Long CL and Humphrey MB, 2012).
Fig. 1. Osteoclast precursor development and differentiation (Long CL and Humphrey MB, 2012).
Effect of APC and Thrombin on Osteoclast Differentiation
Bone remodeling is dynamically accompanied by the action of osteoblasts and osteoclasts, but the contribution of the coagulation system to this process is not fully understood. Activated protein C (APC) was reported to have a positive effect on osteoblast proliferation in a previous study. Yoshida et al. further elucidated the relationship between bone remodeling and blood coagulation by examining the effect of APC on osteoclast differentiation.
Initially, they investigated the influence of APC on osteoclast differentiation induced by receptor activator of nuclear factor-kB ligand (RANKL) and macrophage-colony-stimulating factor (M-CSF). As depicted in Fig. 1A and B, APC dose-dependently inhibited the generation of TRAP-positive multinucleated cells from human osteoclast precursors. APC at a low concentration (<20 nM), such as 4 nM, had no effect. In contrast, the zymogen form of protein C had no impact, and APC did not induce apoptosis in precursor cells and osteoclasts. As shown in Fig. 1C, thrombin also had no effect, even at a low concentration of 1 nM. These results demonstrate that APC specifically suppresses osteoclast differentiation.
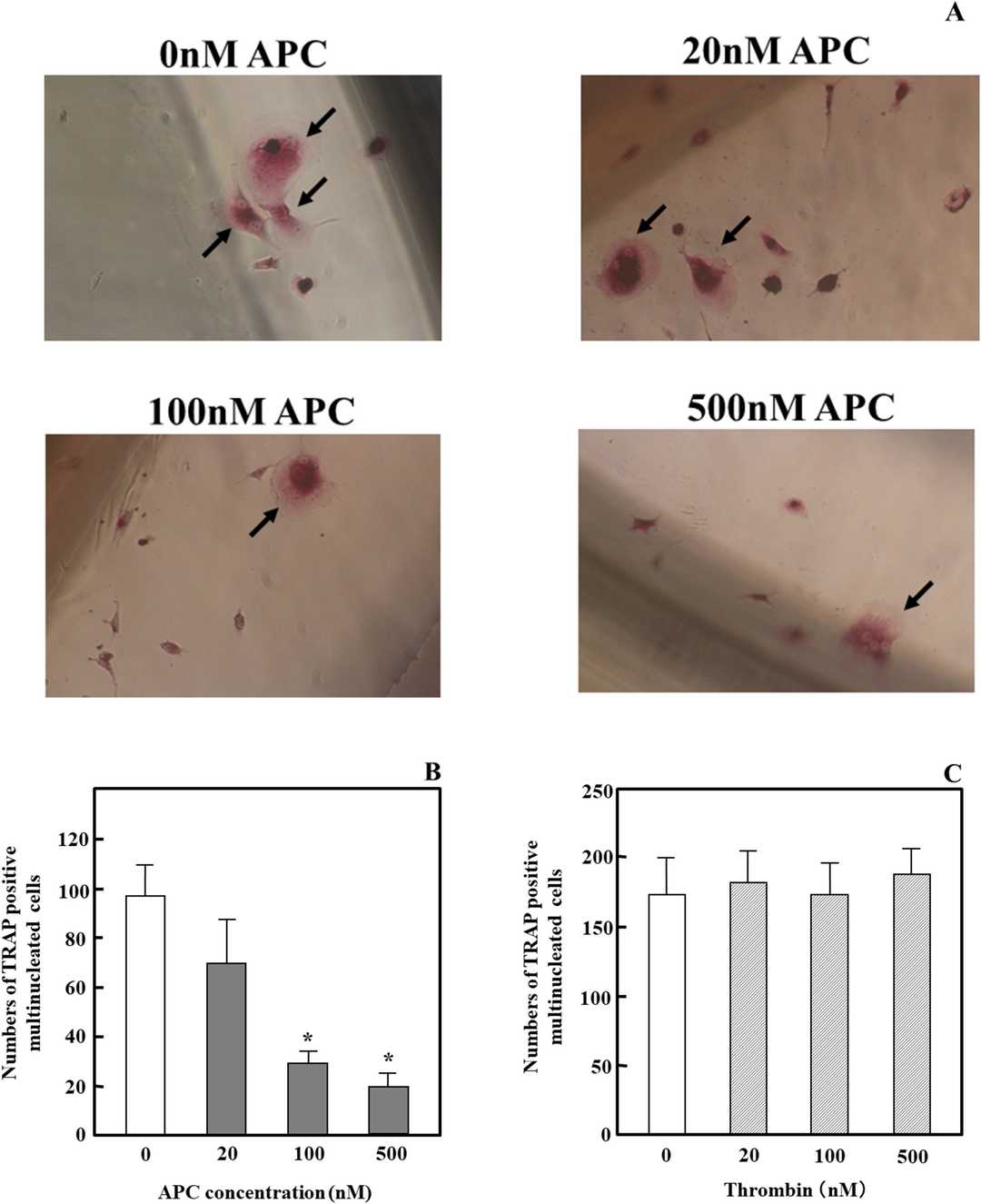 Fig. 1. Effects of APC and thrombin on RANKL- and M-CSF-dependent osteoclast differentiation in osteoclast precursor cells and osteoclasts (Yoshida K, Akita N, et al., 2018).
Fig. 1. Effects of APC and thrombin on RANKL- and M-CSF-dependent osteoclast differentiation in osteoclast precursor cells and osteoclasts (Yoshida K, Akita N, et al., 2018).
THOC5 Dynamically Shuttles between Nucleus and Cytoplasm by c-FMS Signaling
Osteoclasts are essential for bone resorption and originate from myeloid precursors. Their differentiation depends on M-CSF and RANKL signaling. THOC5, a component of the THO complex, is known to regulate macrophage differentiation and hematopoiesis. However, its role in osteoclastogenesis remains unclear.
To explore THOC5's role in human osteoclast precursor cells (OCPs), Mun et al. evaluated its expression. THOC5 was constantly present in human OCPs for up to 48 hours post M-CSF treatment (Fig. 2A). Known as a shuttle protein for mRNA and nuclear proteins, they examined THOC5's localization. THOC5 appeared in both the nucleus and cytoplasm following M-CSF stimulation (Fig. 2B). Cytoplasmic THOC5 levels fluctuated, reducing to baseline levels 48 hours after stimulation (Fig. 2B). Conversely, nuclear THOC5 decreased at 24 hours but rose again at 48 hours post-stimulation. To test if c-FMS signaling affected THOC5's localization, they used Imatinib, a tyrosine receptor inhibitor, to block c-FMS before M-CSF stimulation. Imatinib reversed the nuclear THOC5 increase at 48 hours (Fig. 2C and D). Further, using immunofluorescence microscopy, they observed THOC5 primarily in the nucleus on day 2, spreading to both the nucleus and cytoplasm by day 3, and showing a vesicle-like structure in the cytoplasm by day 5. These indicate THOC5 shuttles between the nucleus and cytoplasm in a M-CSF-dependent manner.
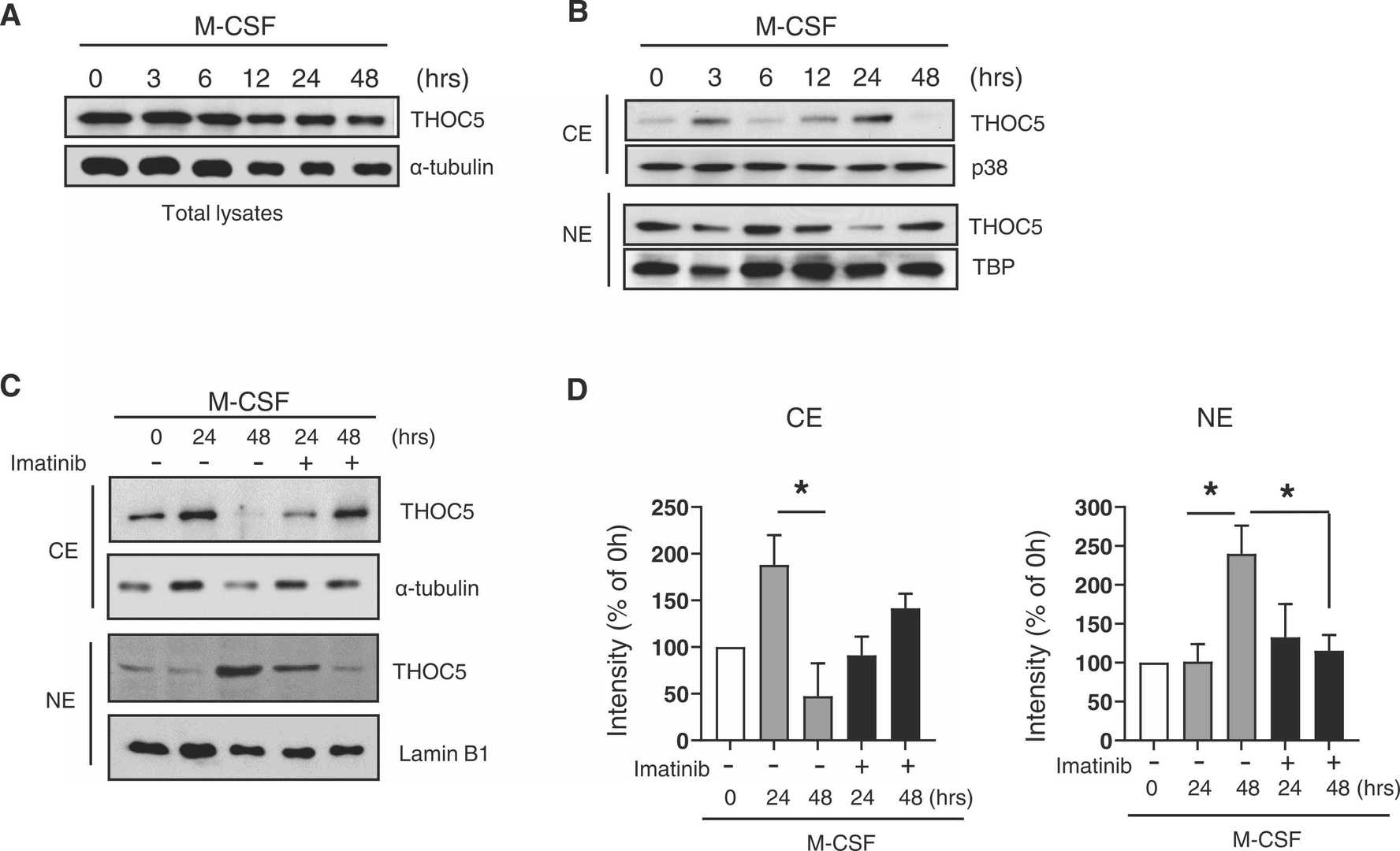 Fig. 2. Subcellular localizations of THOC5 were dynamically regulated during osteoclastogenesis (Mun S H, Oh B, et al., 2022).
Fig. 2. Subcellular localizations of THOC5 were dynamically regulated during osteoclastogenesis (Mun S H, Oh B, et al., 2022).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells