Soft Agar Colony Formation Assay
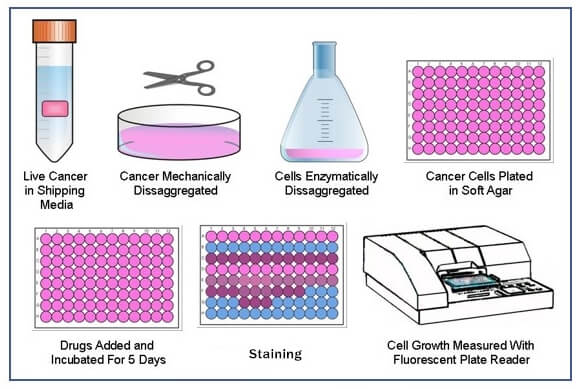
Soft agar colony formation assay is a well-established method to assess the anchorage-independent growth ability of cells for detecting the tumorigenic potential of malignant tumor cells, which is evolved from the plate colony formation assay where cells are seeded on a culture plate to measure the ability of cells to form colonies. The limitation of plate colony formation assay is that it only provides information regarding anchorage-dependent growth. However, malignant cells have the capability to proliferate and grow without attachment to a substrate. Therefore, soft agar colony formation assay is developed to characterize this ability in vitro.
The soft agar colony formation assay has been widely used in cell differentiation, transformation, and tumorigenesis studies as well as in the efficacy evaluation of anti-tumor therapeutics.
Materials
Cell culture medium
Cell culture dishes/plates
Trypsin-EDTA
Centrifuge
Agar
Cell counter
Microscopy
Gel count colony counter
Phosphate buffered saline (PBS)
Table 1. The suggested amounts for soft agar colony formation assay.
| Culture dishes/plates | 96-well | 48-well | 14-well | 6-well | 35 mm | 60 mm | 100 mm |
| Base and top agar volume (mL/well) | 0.1 | 0.2 | 0.5 | 1.0 | 1.5 | 3.0 | 5.0 |
| Cells/well | 500 | 1,000 | 1,250 | 2,500 | 5,000 | 7,500 | 12,500 |
| Media volume for feeding (mL/well) | 0.05 | 0.1 | 0.25 | 0.5 | 0.75 | 1.5 | 2.5 |
Assay Procedure
- Preparation of agar solution
1) Preparing 5% agar solution by dissolve 5 g agar powder in 100 mL of deionized water, autoclave at 121°C for 15 min.
Note: These mixtures can be made in advance and store at 4°C but should be heated again at the time of the experiment until agar has completely dissolved. - Production of the bottom layer of agar
1) Add 9 mL complete medium (37°C) to 1 mL 5% agar solution (50°C) and mix thoroughly.
2) Pipette 0.8 mL mixture to each well of 12-well plate and allow it to solidify for 30 min at room temperature. - Preparation of cell suspension
1) Remove the complete medium from culture dish and wash cells with PBS.
2) Add 0.5 mL 0.25% trypsin-EDTA (37°C) for 3-5 min and collect detacted cells by adding complete medium.
3) Spin cells at 600 g for 5 min and resuspend cells in complete medium, then count cells and adjust the concentration of cells to 1 x 103 cells/mL. - Production of the upper layer of agar
1) Add 9.4 mL resuspended cells (37°C) to 0.6 mL 5% agar solution (50°C) and mix homogeneously.
Note: Pay attention to the temperature of agar solution and complete medium. It is recommended to keep agar solution and complete medium at 50°C and 37°C, respectively, and mix them as soon as possible to avoid in-homogenous agglomeration.
2) Pipette 0.8 mL the cell/agar mixture onto the solidified bottom layer of agar in 12-well plate and allow it to solidify for 30 min at room temperature.
Note: 1) Do not pour the upper layer of agar until the bottom layer of agar is coagulated completely. 2) Use caution to avoid deposition of any air bubbles into the plate wells.
3) Add 800 μL complete medium on top to prevent drying of agar and then cells are maintained in a 37°C humidified incubator with 5% CO2. - Clone counting
1) Monitor colony formation for 2-3 weeks before counting.
Note: Adjust the incubation period according to the tumorgenecity of the cell line.
2) Count all the colonies per well with a gel count colony counter, and then determine the average number of colonies of the three replicates for each group.
3) Capture images of colonies at room temperature using a microscopy.
References
- Stanley Borowicz, et al.; The soft ager colony formation assay. J Vis Exp, 2014, 92: 51998.
- Fedr R, et al.; Automatic cell cloning assay for determining the clonogenic capacity of cancer and cancer stem-like cells. Cytometry A, 2013, 83(5): 472-482.
Cell Services:
Cell Line Testing and Assays: