COLO-818
Cat.No.: CSC-C0237
Species: Homo sapiens (Human)
Source: Hypodermis Metastasis
Morphology: adherent growth in monolayers, mostly spindle-like, some cells with cellular processes, 1-5% very big cells, no strict contact inhibition
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: cytokeratin -, desmin -, endothel -, GFAP -, HMB-45 -, neurofilament -, vimentin +
Viruses: ELISA: reverse transcriptase negative; PCR: EBV -, HBV -, HCV -, HIV -,
The COLO-818 cell line is an immortalized human malignant melanoma cell line first established in 1980 from a cutaneous metastatic lesion of the 50-year-old female patient, with a primary nodular melanoma (most aggressive clinical subtype). The parental cells of this cell line were originally epidermal melanocytes and preserved basic malignant characteristics of late-stage melanoma. The cells are phenotypically polymorphic in vitro, they are mainly spindle-shaped or polygonal and grow partly in semi-suspension. There is a large amount of brown/black melanin granules in the cytoplasm, large irregular nucleus and high incidence of multinucleation. COLO-818 contains the BRAF V600E hotspot mutation that activates the MAPK/ERK signaling pathway and expresses melanoma-specific proteins including TYR, MITF, and gp100.
Functionally, it retains the ability to synthesize melanin, high invasiveness (better Transwell matrix penetration), and also drug resistance to traditional chemotherapy drugs (such as dacarbazine) by overexpression of ABC transporters. It is commonly used in basic research on melanoma to provide a cell model for understanding BRAF-mediated oncogenesis, melanin synthesis and regulation, as well as to investigate the mechanism of chemotherapy drug resistance, and to provide support for the validation of novel therapies such as BRAF/MEK inhibitors and gp100-CAR-T cells.
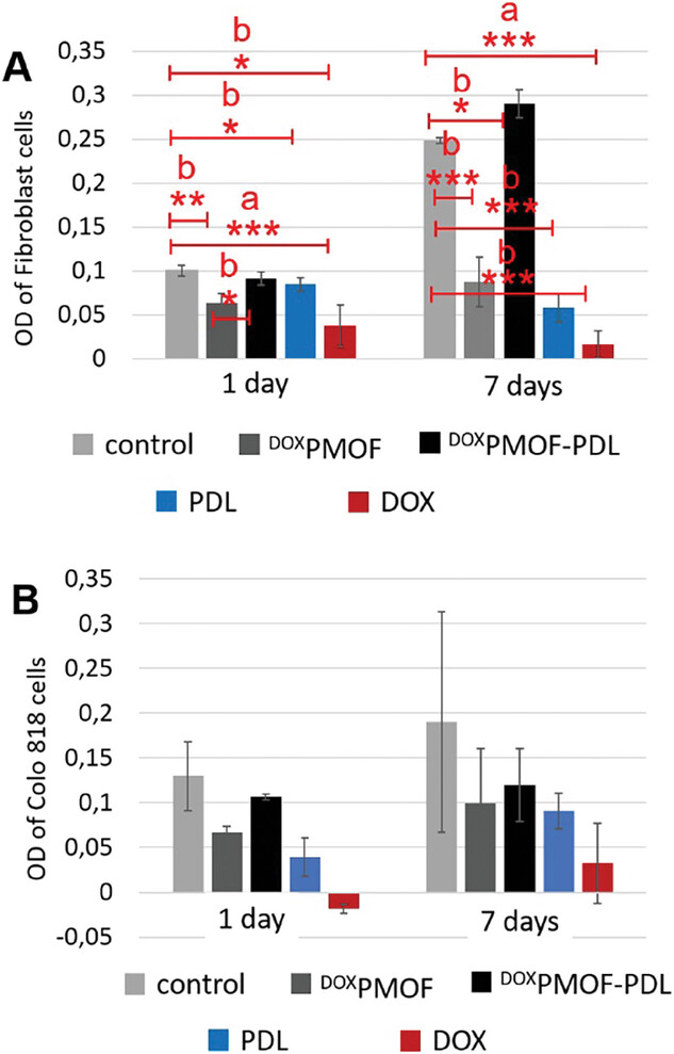
Cell Viability Experiment on DOXPMOF, DOXPMOF-PDL, Free-PDL, and Free-DOX
Solid tumors often suffer from hypoxia, which promotes cancer cell aggressiveness and tissue damage. Therefore, a pH-responsive nanomaterial that co-delivers oxygen and anticancer drug was synthesized. The material offers 14 days of sustained oxygen release and 30 days of pH-dependent drug release under hypoxic conditions.
In a one-pot synthesis, Kehr et al. integrated oxygen-carrying PFC units into the PMO network (PMOF) due to PFC's oxygen-dissolving capacity. The PMOF pores were loaded with DOX for drug delivery and then coated with PDL to create pH-responsive DOXPMOF-PDL. To assess the impact on cell viability, they treated fibroblast and Colo 818 cells with DOXPMOF, DOXPMOF-PDL, free-PDL, and free-DOX. The cell viabilities were then quantified after 1 and 7 days (Fig. 1). The results showed that DOX and PDL alone reduced the cell viabilities of both normal and cancer cells. In most other cases, the immobilization of DOX and PDL on PMOF improved cell viabilities. Of note, DOXPMOF-PDL increased the healthy cell viability by 15%, 79%, and 95% compared to cell culture plate, free-PDL and free-DOX, respectively, and decreased the cancer cell viability by 50% compared to cell culture plate, and increased the viability by 25% and 73% compared to free-PDL and free-DOX after 7 days. In addition, healthy cells within DOXPMOF-PDL sample showed 59% higher viability than cancer cells after 7 days. In conclusion, immobilizing DOX and PDL on PMOF reduced the inhibitory effects of both molecules on the cell viability, especially in healthy cells due to higher DOX release in the acidic environment of cancer cells.
Cell Experiments in the GA5, GA10, and GA10-P Hydrogels
Existing biomaterials frequently lack multi-functionality and precise control of cellular behavior. Motealleh et al. presented a novel step-gradient composite hydrogel (GradGA) 3D bioprinted with a multifunctional NM, Alg and gelatin methacryloyl, with the aim of fabricating a biomaterial that can support healthy cell viability and migration, but not cancer cell growth, by utilizing pH-responsive drug release.
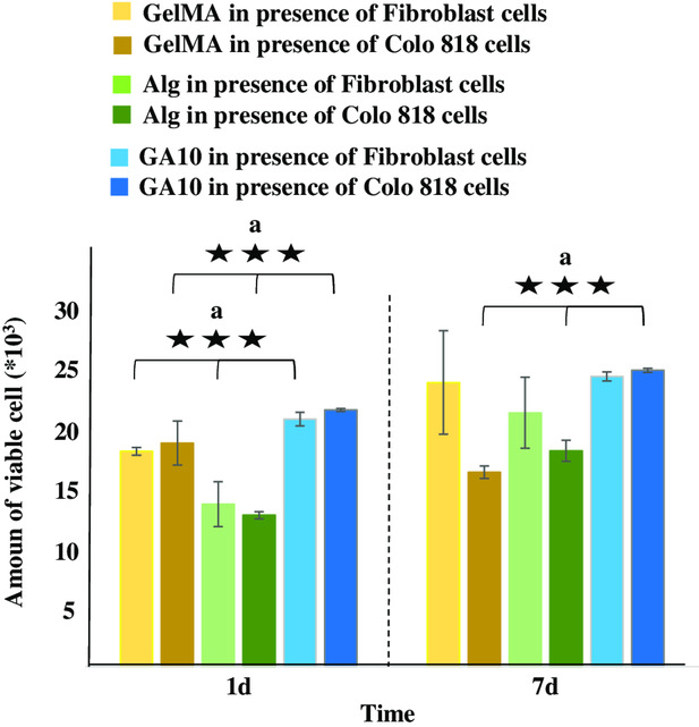
Cell experiments were performed to compare the effects of GelMA, Alg, and the composite (GA10) on cell viability, confirming the advantage of the addition of GelMA into Alg. GelMA, Alg, and GA10 were separately incubated with healthy dermal fibroblasts and malignant melanoma fibroblasts (Colo 818) for 1 and 7 days, after which cell viability was determined using PrestoBlue (Fig. 2). Data revealed higher cell viability in GA10 over GelMA or Alg, especially after 1 day. This suggested a synergistic effect of GelMA and Alg on cellular viability, leading to further experiments of GelMA/Alg-based composites with different ratios (GA5, GA10 and GA10-P).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells